Use of recombinant human granulocyte macrophagocyte colony stimulating factor in preparation of drug for preventing and treating hepatitis B
A technology of colony stimulating factor and hepatitis B, applied in the field of medicine, can solve problems such as low response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
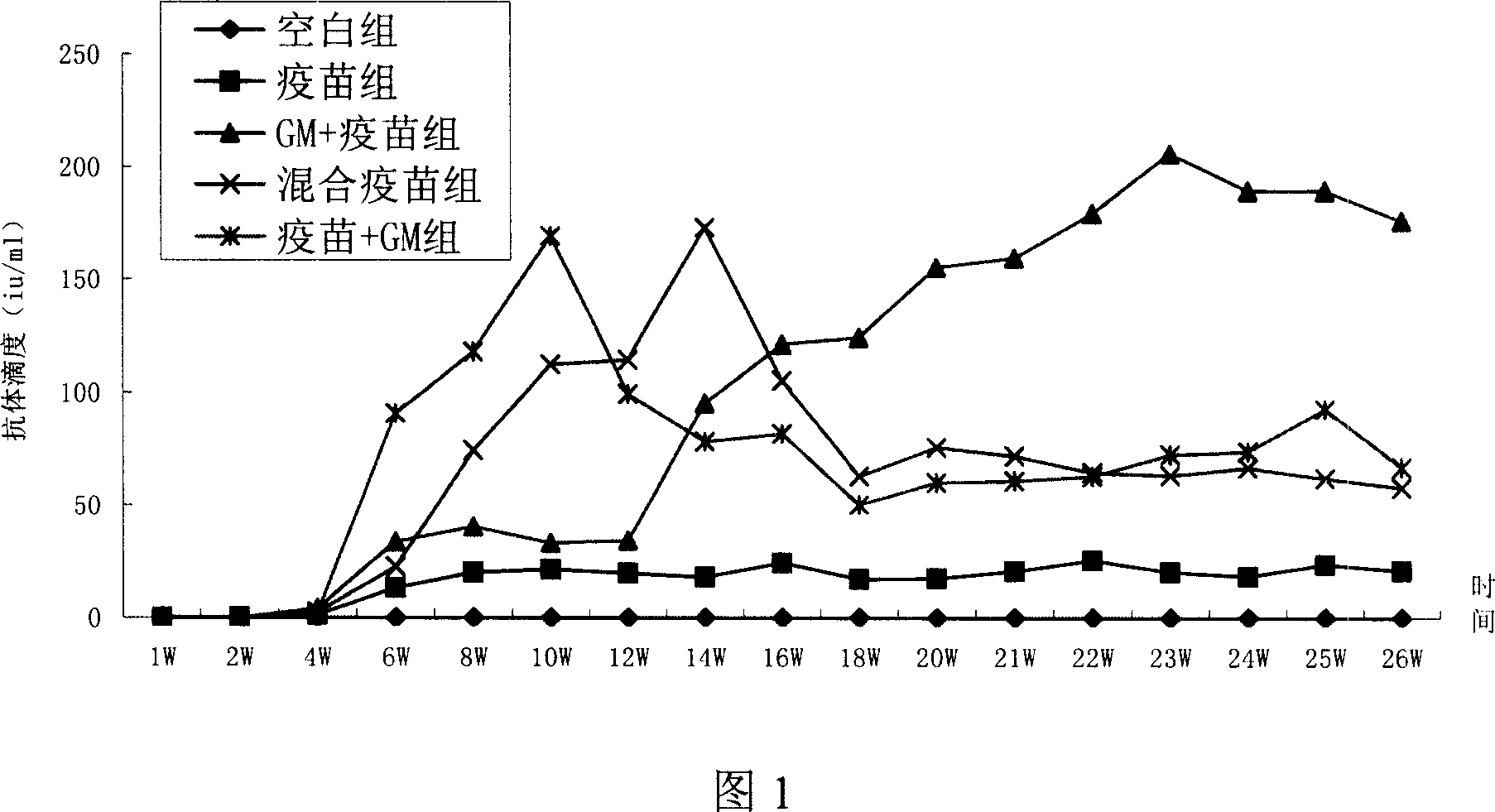
[0020] Experimental Example 1: Recombinant human granulocyte-macrophage colony-stimulating factor and genetically engineered hepatitis B vaccine synergistically induce cellular immune responses in mice Materials and instruments:
[0021] Experimental animals: BALB / C mice, SPF grade, female, weighing 16-18 g, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0022] Experimental materials: Genetic engineering (CHO cell) hepatitis B vaccine [Recombinant Hepatitis BVaccine (CHO)], 10μg / 1.0ml, recombinant human granulocyte macrophage colony-stimulating factor for injection [Recombinant Human Granulocyte / Macrophage Colony-Stimulating Factor for Injection ] (hereafter referred to as rhGm-csf), 300μg / support, the stock solution of genetically engineered hepatitis B vaccine (HBsAg stock solution) expressed by CHO is produced by North China Pharmaceutical Group Jintan Biotechnology Co., Ltd.
[0023] Main reagents and instruments: RPMI 1640 culture medium (...
experiment example 2
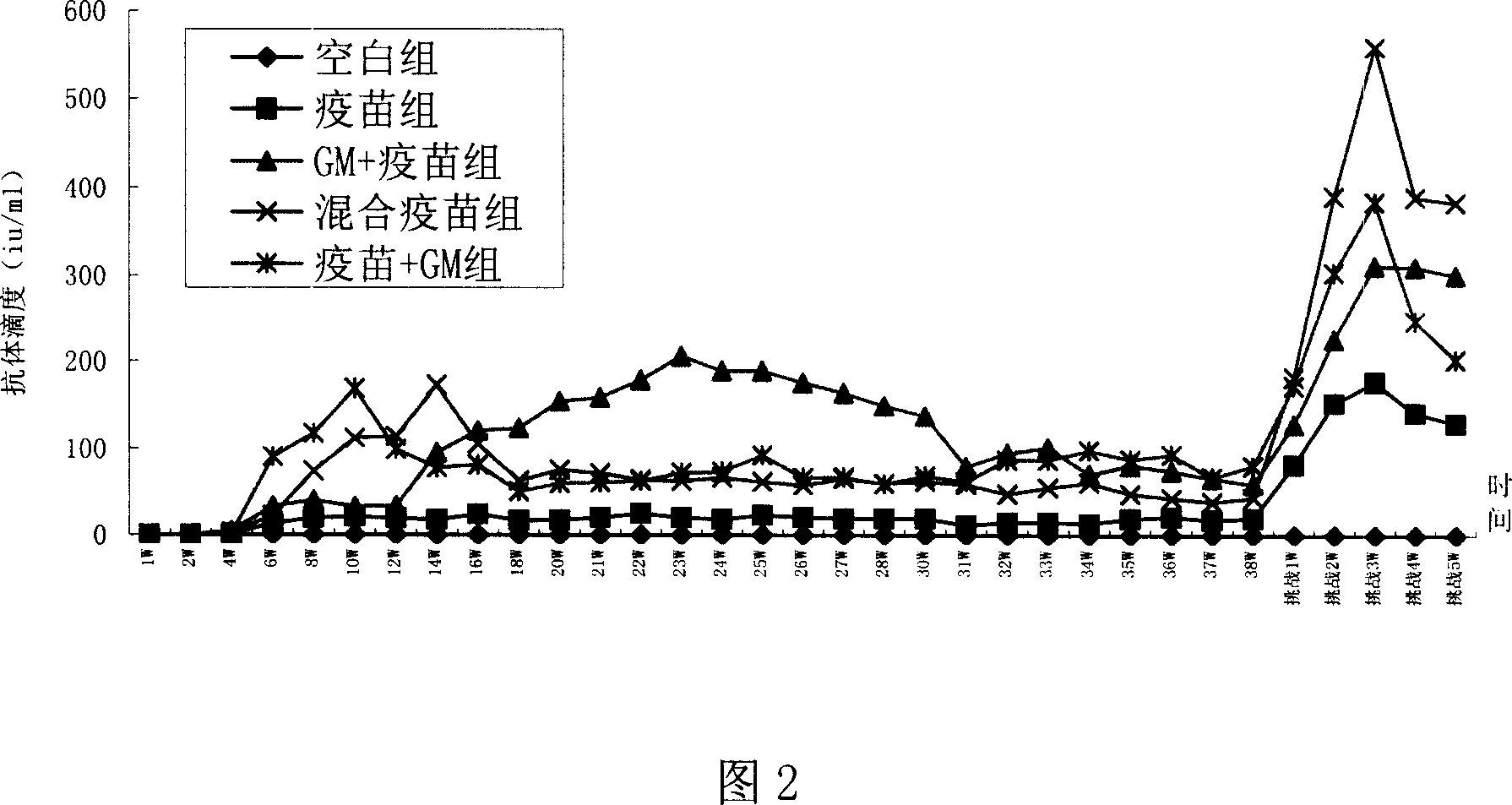
[0076] Experimental Example 2: Recombinant human granulocyte-macrophage colony-stimulating factor and genetically engineered hepatitis B vaccine synergistically induce humoral immune responses in mice
[0077] Materials and Instruments:
[0078] Experimental animals, materials, main reagents and instruments are the same as in Experimental Example 1.
[0079] Detection method:
[0080] 1. Serum antibody titer determination method:
[0081] Using the "Hepatitis B virus surface antibody ELISA diagnostic kit", the OD value of the sample / OD value of the negative control ≥ 2.1 is judged as positive (the OD value of the negative control is less than 0.05, calculated as 0.05), and the specific operation is shown in the instructions .
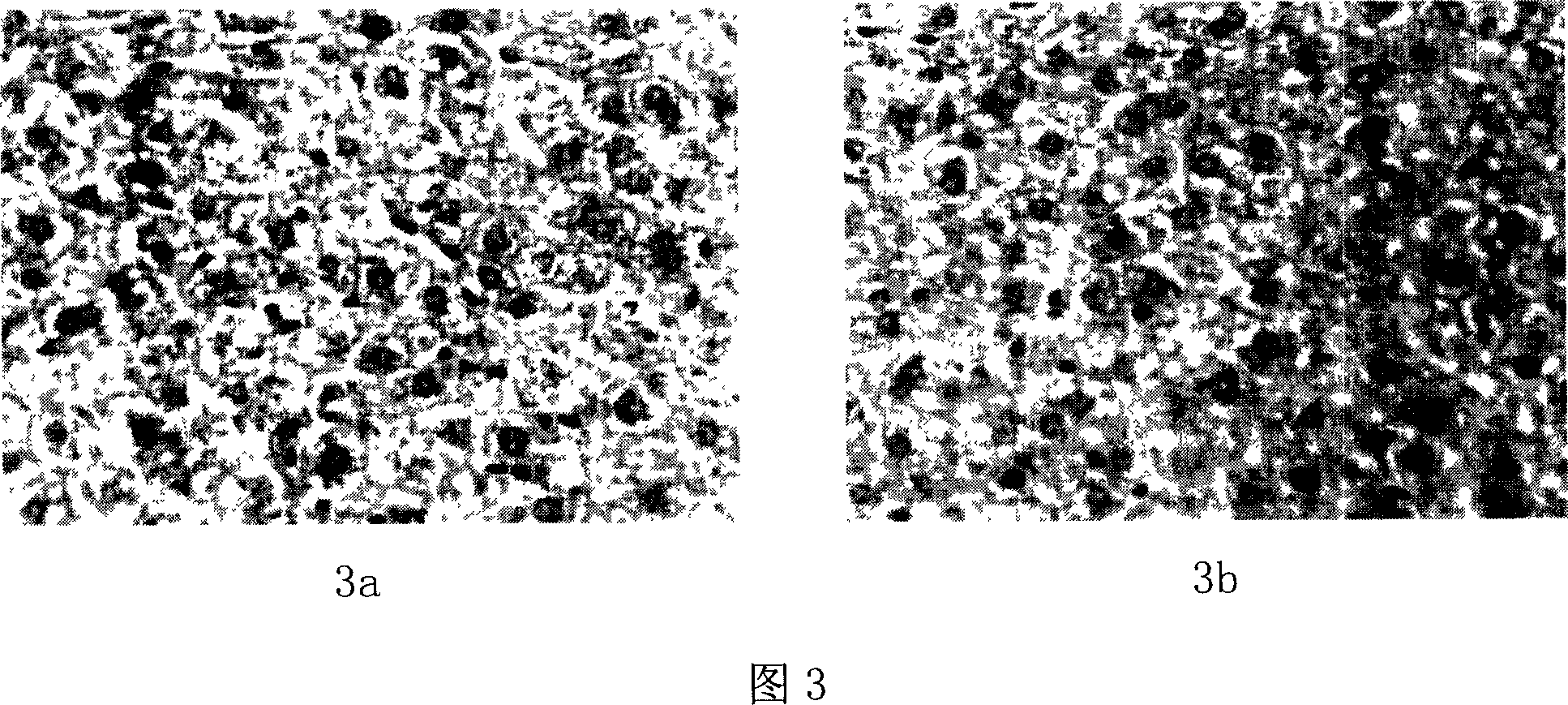
[0082] 2. Liver tissue section examination method (HE staining method):
[0083] After the mice were killed, cut the abdominal cavity with scissors, observe the liver tissue, cut off the middle lobe of the liver tissue, and immediately put it into nor...
experiment example 3
[0110] Experimental Example 3: Comparison of recombinant human granulocyte macrophage colony-stimulating factor and genetically engineered hepatitis B vaccine from three sources synergistically inducing cellular immune responses in mice
[0111] Materials and Instruments:
[0112] Experimental animals, main reagents and instruments are the same as in Experimental Example 1.
[0113] Experimental materials: Genetic engineering (CHO cell) hepatitis B vaccine [Recombinant Hepatitis BVaccine (CHO)], 10μg / 1.0ml, recombinant human granulocyte macrophage colony-stimulating factor for injection [Recombinant Human Granulocyte / Macrophage Colony-Stimulating Factor for Injection ] (hereinafter referred to as rhGm-csf), 300 μg / support, the genetically engineered hepatitis B vaccine stock solution (HBsAg stock solution) expressed by CHO is all produced by North China Pharmaceutical Group Jintan Biotechnology Co., Ltd.; recombinant (Hansenula) hepatitis B vaccine [Recombinant Hepatitis B Va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com