Purification method of recombinant yeast strain and rhGM-CSF to express human granulocyte-macrophage colony stimulating factor
A colony-stimulating factor and macrophage technology, which is applied in the field of yeast recombinant strain expressing human granulocyte-macrophage colony-stimulating factor and the purification of rhGM-CSF, can solve the problems of easy gene loss, unstable expression and large side effects. , to achieve the effect of reducing separation and purification steps, reducing clinical side effects and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
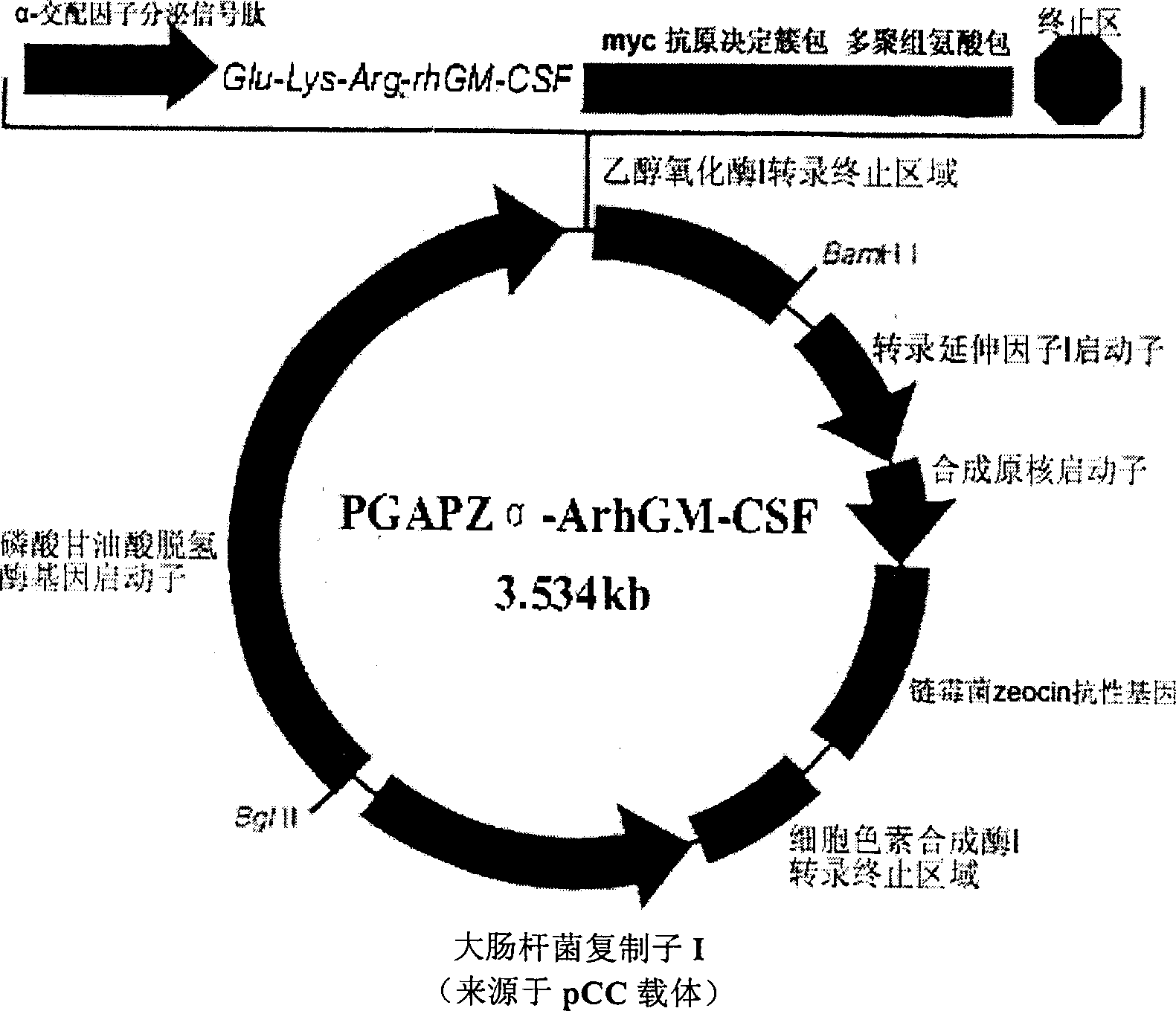
[0033] 1. Construction of rhGM-CSF yeast engineering bacteria
[0034] (1) Materials:
[0035] 1. rhGM-CSF gene
[0036] 2. pGAPZα-A and P. pastoris Strain GS115 (his4) were purchased from Invitrogen, Australia.
[0037] (2) Method:
[0038] 1. Gene PCR amplification:
[0039] (1) Primer design: delete the Ste13 site (Glu-Ala-Glu-Ala) and the initiation codon ATG after the Kex2 (Lys-Arg) site in the 5' end primer.
[0040] P1-5'G CTCGAG AAAAGA ATG GCTCCAGCCCGT3' ("ATG" is removed)
[0041] XhoI Lys-Arg (Kex2 site)
[0042] P2-5'G TCTAGA TTACTCCTGGACTGGCTC3'
[0043] wxya
[0044] (2) Thermal cycle: 95°C, 5'; 95°C, 30"→43°C, 30"→72°C, 40"; 72°C, 10'; 30 cycles of amplification.
[0045] 2. PCR amplification product recovery and cloning:
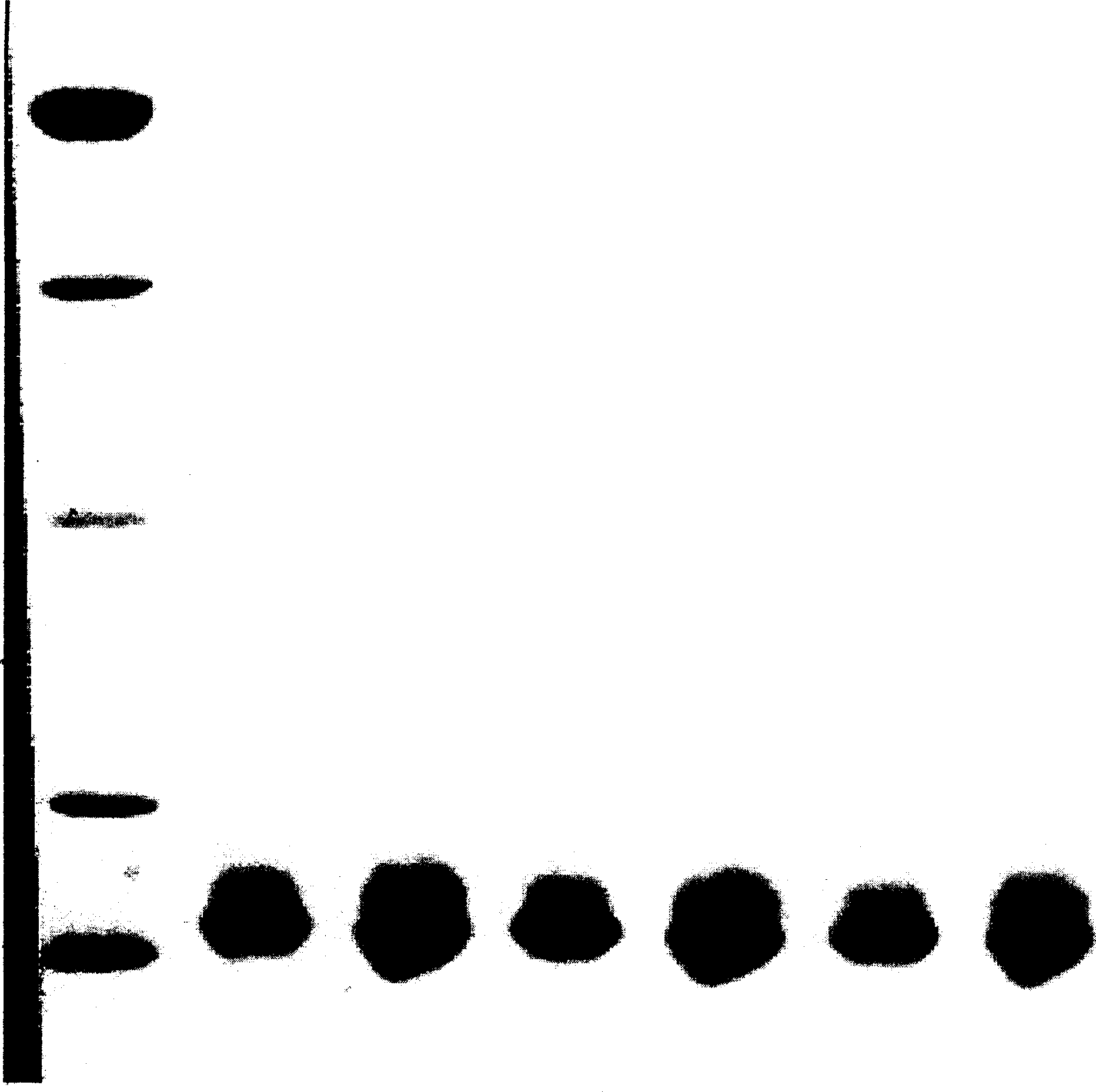
[0046] (1) The rhGM-CSF gene was amplified and electrophoresed to obtain a DNA band of about 400 bp;
[0047] (2) The target fragment was recovered with a high-purity kit for PCR products prod...
Embodiment 2
[0052] Purification method of rhGM-CSF:
[0053] 1. Strain storage
[0054] After constructing highly efficient rhGM-CSF-expressing yeast engineered bacteria (rhGM-CSF / pGAPZα-A / GS115), the strains were stored at -80°C, or the skim milk was freeze-dried and then sealed.
[0055] 2. Activation of strains
[0056] Plate medium formula (%):
[0057] Yeast powder 1.0 Peptone 2.0 Glucose 2.0 Agar 2.0
[0058] Take a bacterial strain, pick the bacterial liquid to scratch the plate, and then culture it at 30°C for 24 hours
[0059] 3. First-class seed liquid
[0060] YPD medium formula (%):
[0061] Yeast powder 1.0 peptone 2.0 glucose 2.0
[0062] Add 100mg / Lzeocin to the YPD medium, then take the activated bacterial solution to inoculate at 1% of the inoculum size, and incubate at 30°C for 12 hours.
[0063] 4. Secondary seed solution
[0064] YPD medium formula (%):
[0065] Yeast powder 1.0 peptone 2.0 glucose 2.0
[0066] Inoculate at 1% of the inoculum size and incubat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com