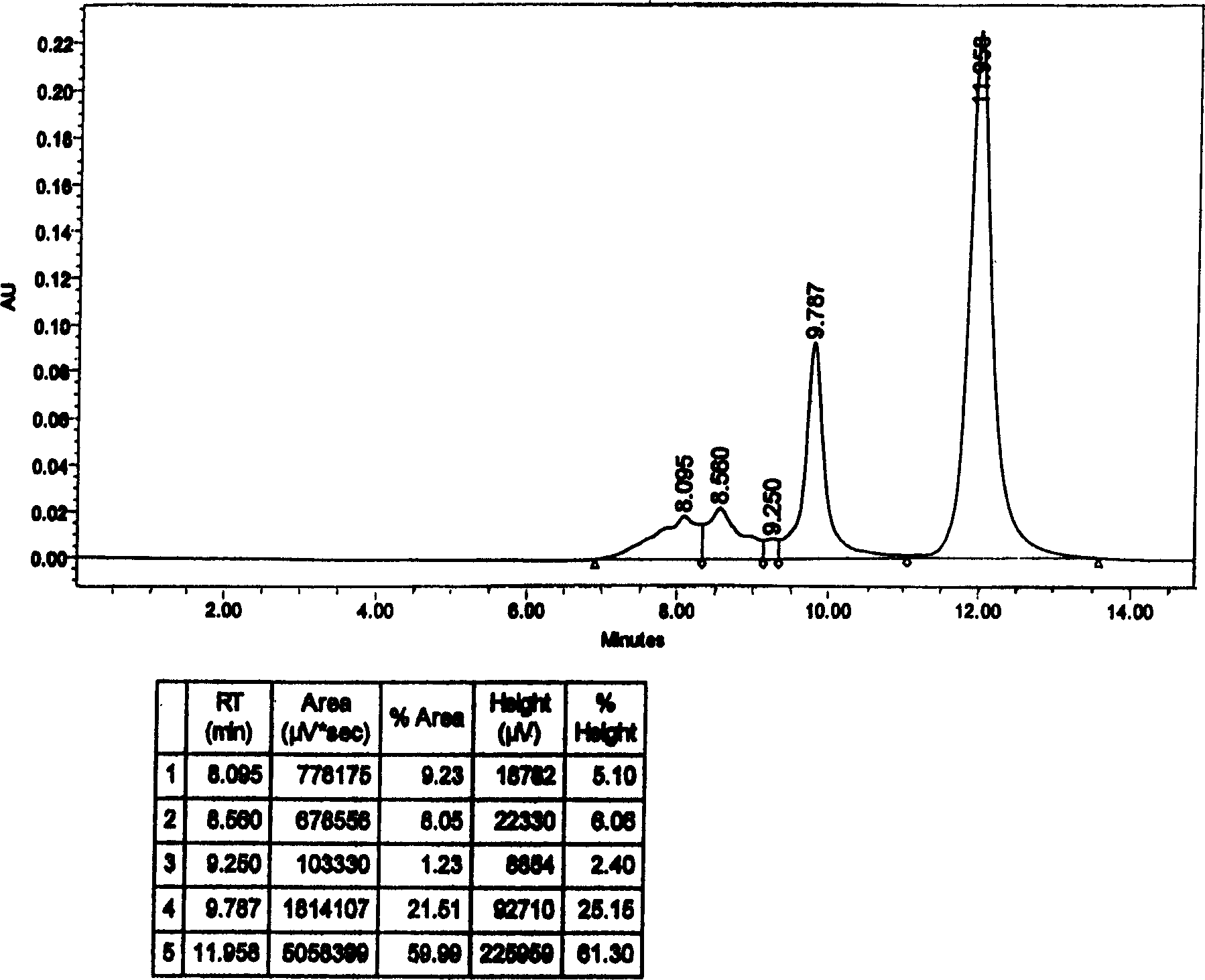
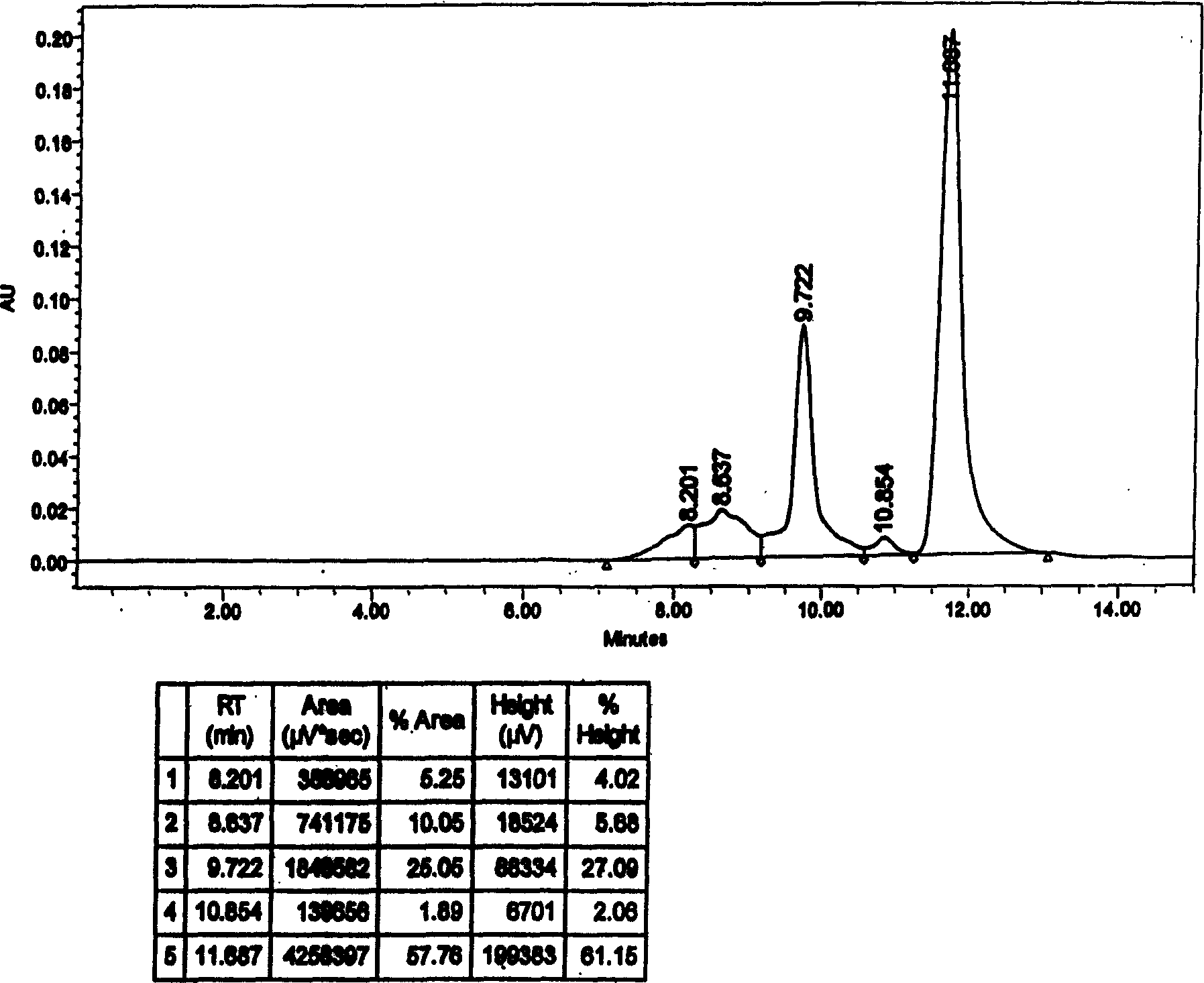
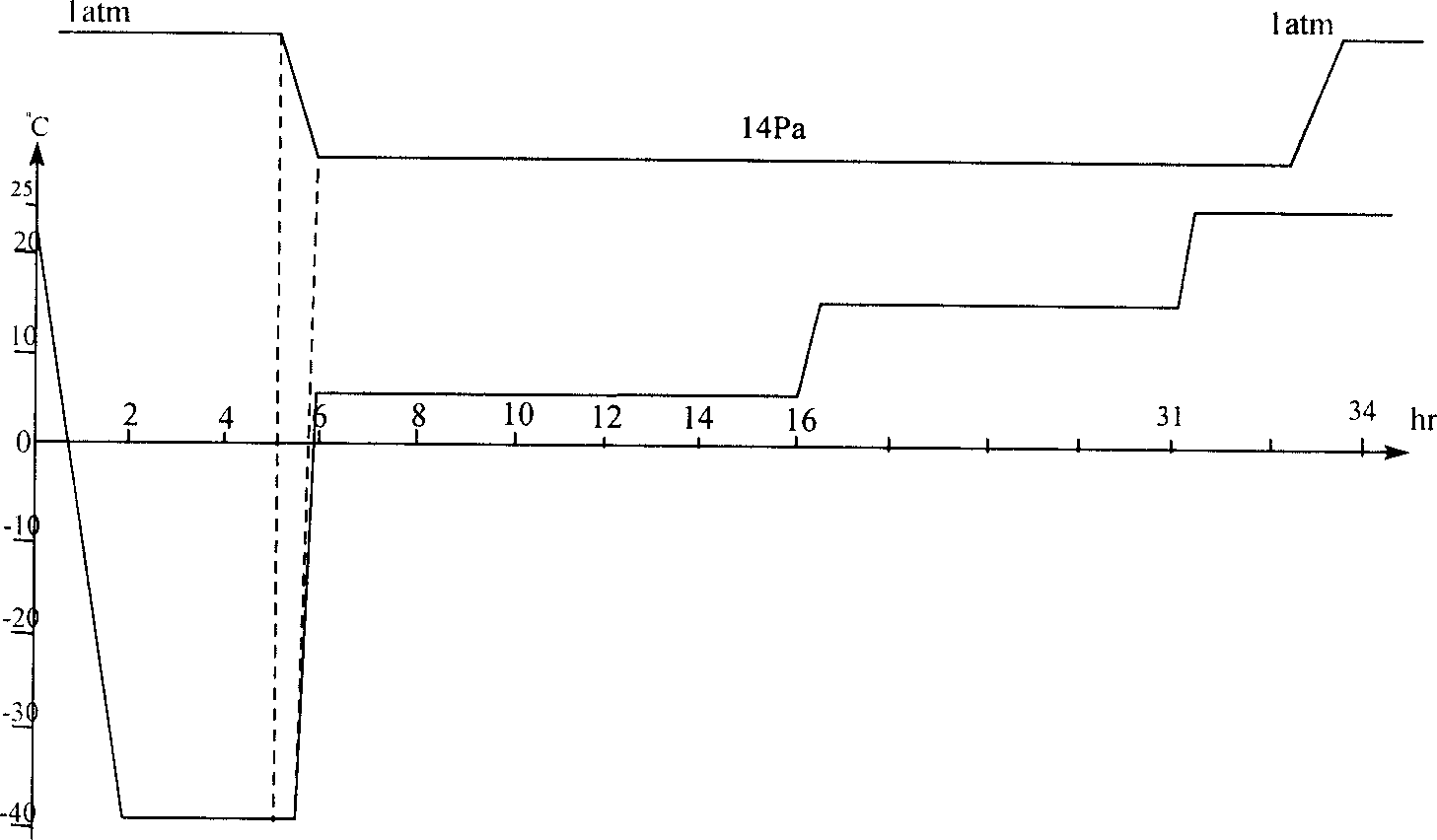
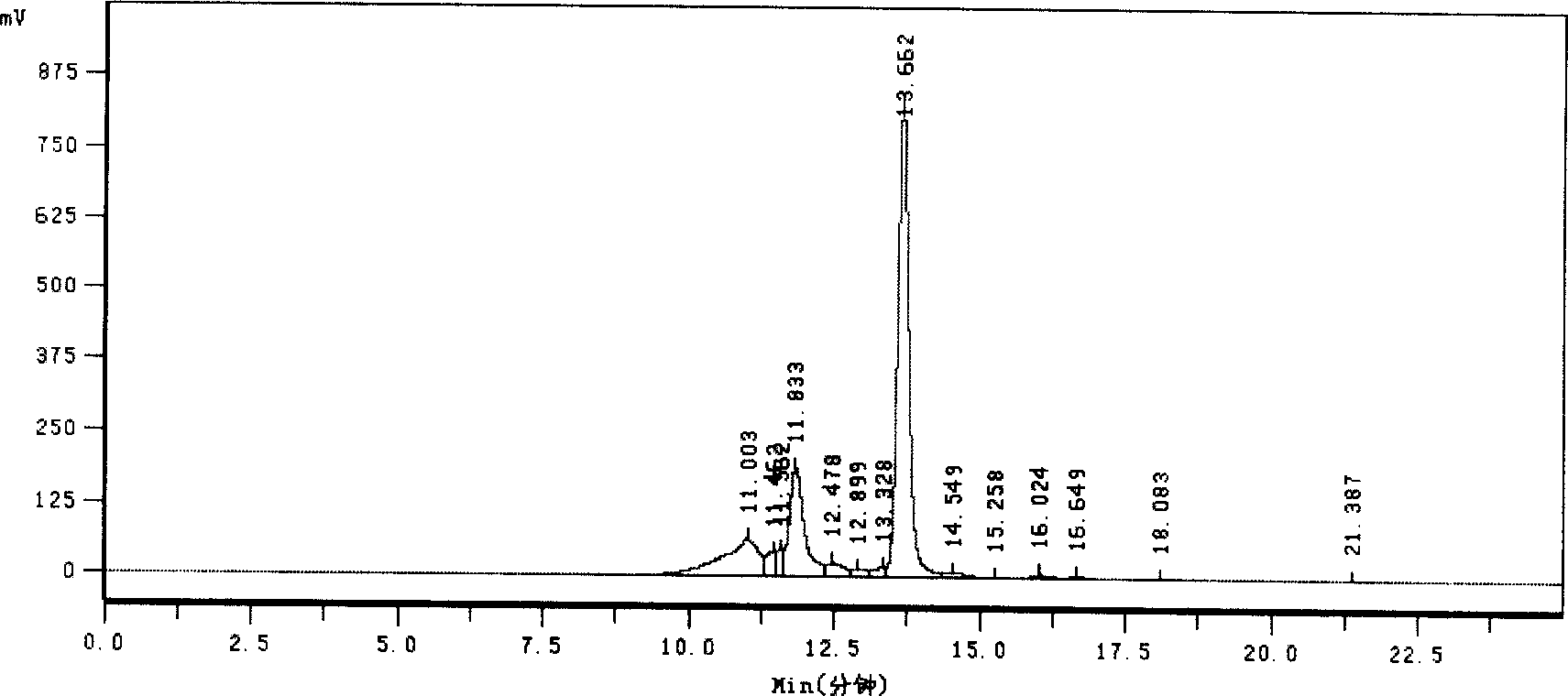
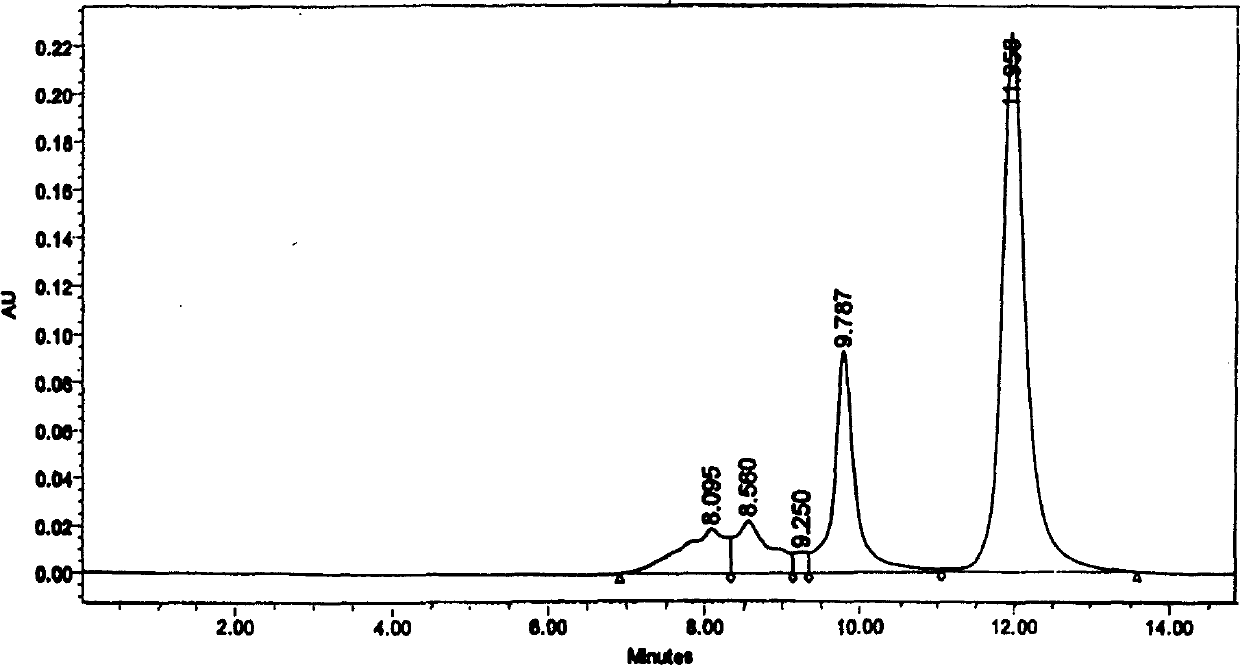
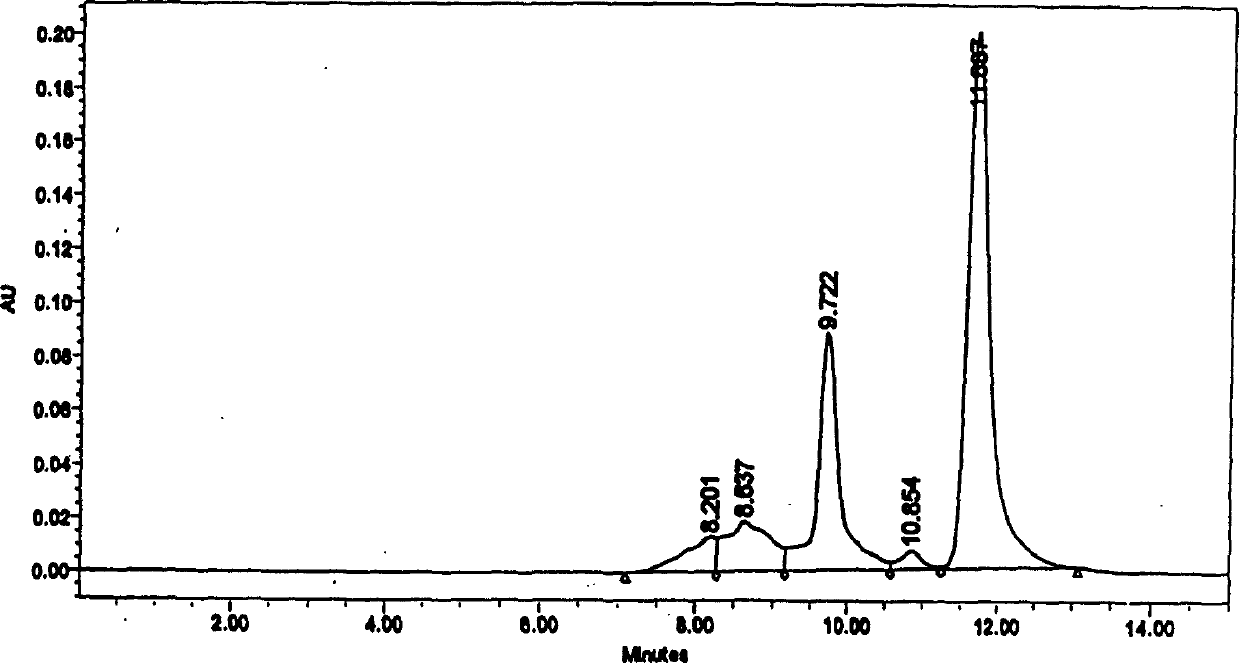
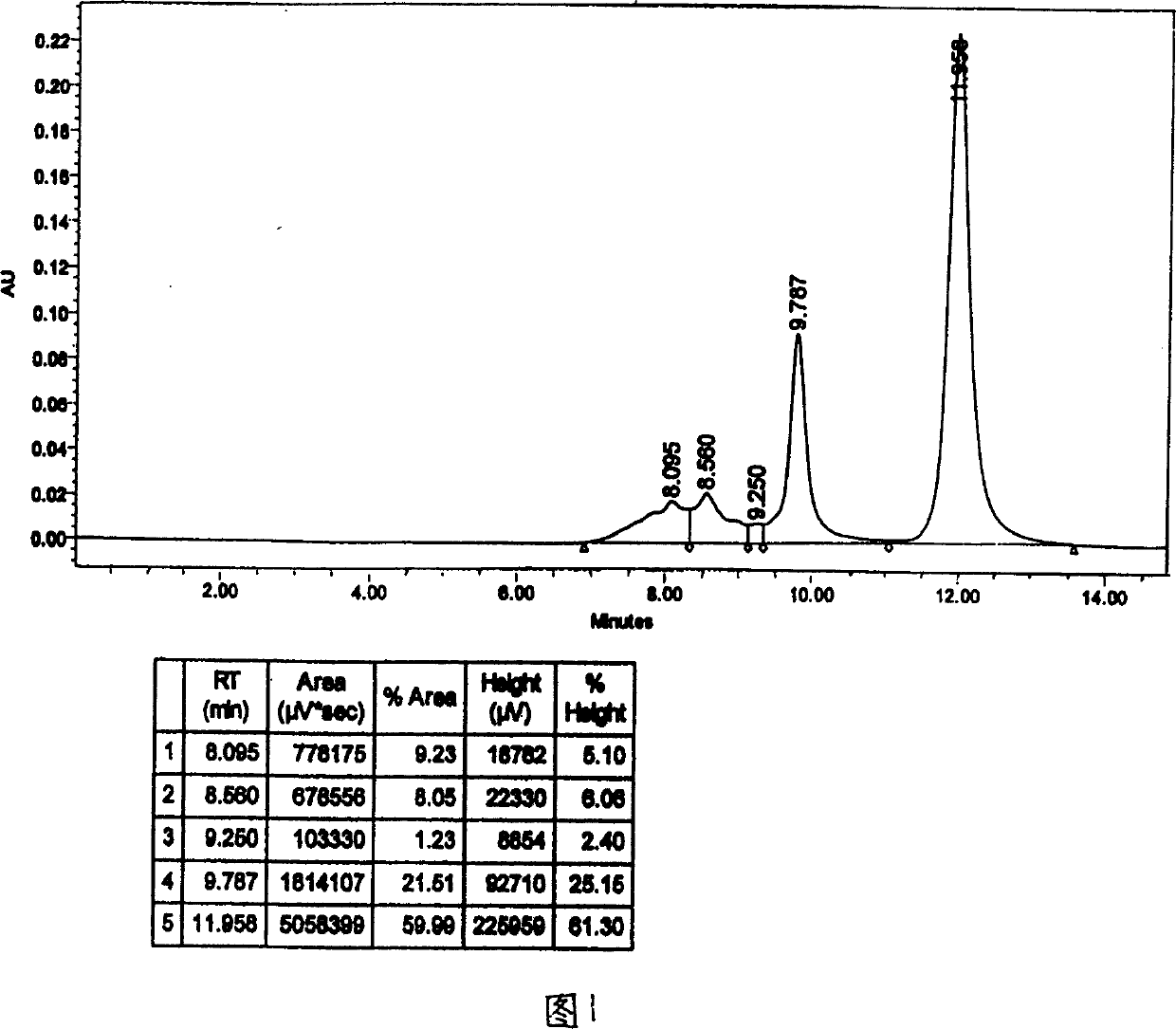
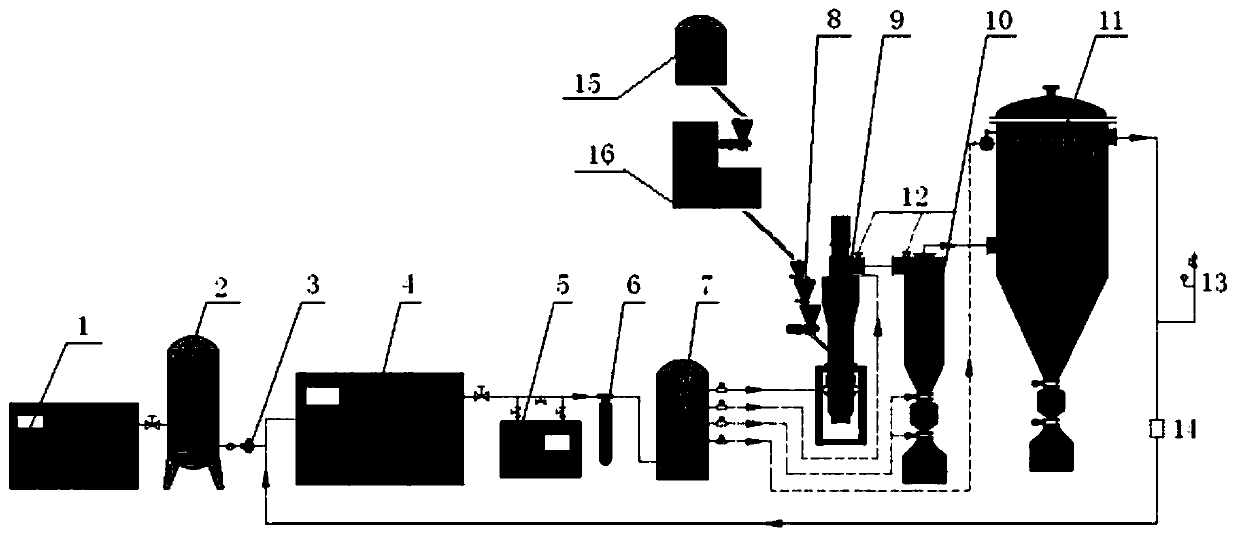
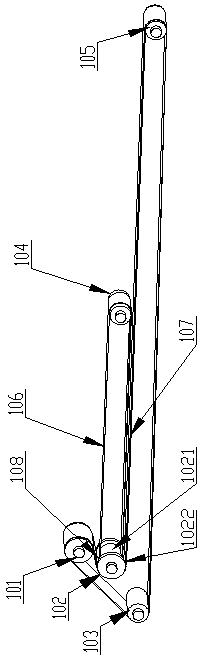
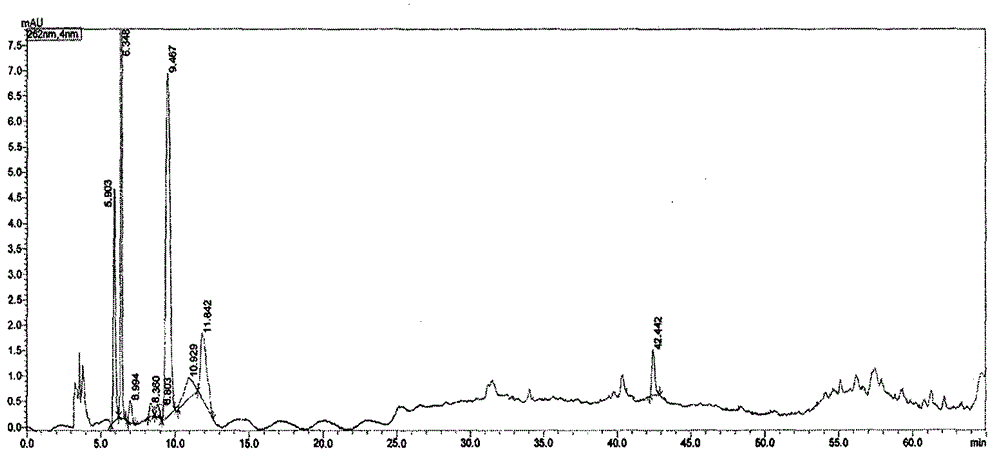
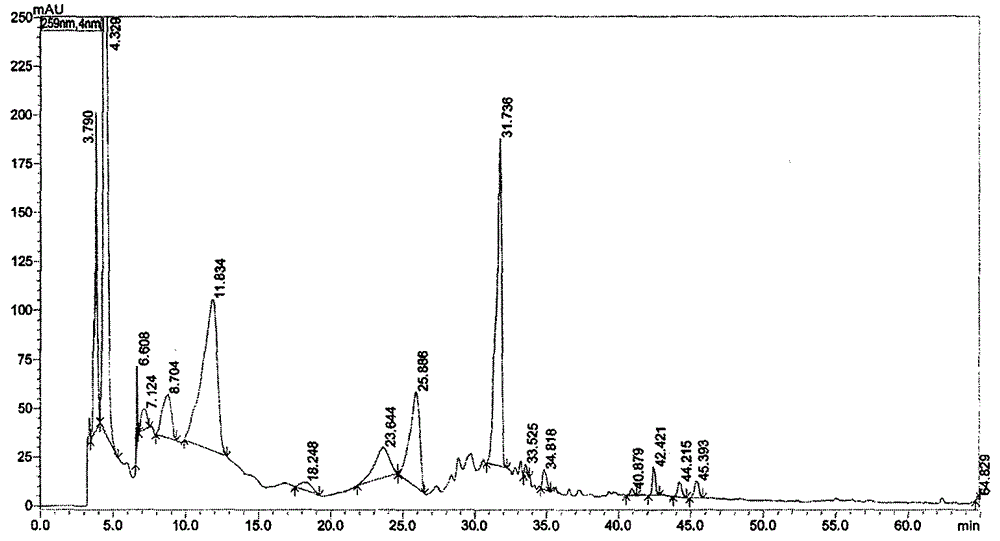
Patents
Literature
107results about How to "Comply with GMP requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
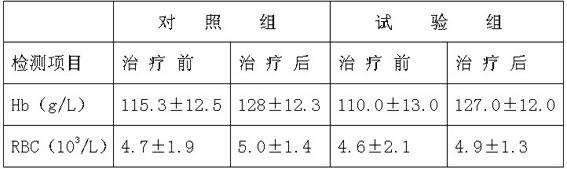
Method for preparing pharmaceutics of hydrolysate of brain protein
ActiveCN1562339ATo achieve the separation effectNo pollution in the processPowder deliveryNervous disorderHydrolysateTyrosine
A hydrate of brain protein is prepared from pig's brain through adding purified water, homogenizing, heating, cooling, regulating pH=1.5-2.0, enzymolyzing, regulating pH=7.7-8.0, enzymolyzing, regulating pH=2.5-3.0 freezing, thawing, filter, regulating pH to become neutral, ultrafiltering, concentrating, sterilizing, adding amino acids, regulating peptide map, diluting, and steam sterilizing.
Owner:赛隆药业集团股份有限公司
Brain protein hydrolysate and production process of its freeze dried preparation
InactiveCN1857711ANo pollution in the processReduce investmentPowder deliveryNervous disorderFreeze-dryingNitrogen
The present invention relates to a kind of brain protein hydrolysate and the production process of its freeze dried preparation. The brain protein hydrolysate for injection is prepared with pig brain and through the steps of homogenating in a colloid mill to collect slurry, hydrolyzing with pepsase and pancreatin to collect supernatant, filtering with filer paper to collect filtrate, separating and purifying the filtrate with hydroxyapetite column, regulating peptide map and collecting object, ultrafiltering with membrane of intercepting molecular weight 8 KD to collecting filtrate, nitrogen and amino acid analysis and adding amino acid in the required amount, and fine filtering with 0.22 micron filtering membrane. The brain protein hydrolysate for injection may be further freeze dried to obtain freeze dried brain protein hydrolysate preparation. The production process is environment friendly, high in yield and low in cost.
Owner:HAINAN JINXING PHARMA
Dendrobium candidum ganoderma lucidum capsule
ActiveCN102772672ANo pollution in the processNarrow particle size distributionMetabolism disorderAntinoxious agentsDendrobium candidumHigh absorption
The invention discloses a dendrobium candidum ganoderma lucidum capsule, and relates to a traditional Chinese medicine capsule, in particular to a dendrobium candidum ganoderma lucidum capsule free from influence of air humidity and having high absorption and high bioavailability and a preparation method thereof. The dendrobium candidum ganoderma lucidum capsule disclosed by the invention is characterized by being prepared by the four steps of processing of dendrobium candidum, ultrafine grinding of dendrobium candidum, ultrafine grinding of ganoderma lucidum and mixing and capsule filling. In the dendrobium candidum ganoderma lucidum capsule disclosed by the invention, the ultrafine powder of dendrobium candidum and the ultrafine powder of ganoderma lucidum are mixed and packed in a capsule form, the influence of air humidity can be avoided, and the human body absorption and the bioavailability are high; and moreover, the dendrobium candidum ganoderma lucidum capsule has the functions of dendrobium candidum and ganoderma lucidum, and has a better taking effect.
Owner:XISHUANGBANNA ZENGLIANG BIOTECH
Dendrobium officinale healthcare product with auxiliary blood glucose reducing function and preparation method thereof
ActiveCN104784505APromote absorptionImprove playbackMetabolism disorderFood preparationMedicinal herbsSide effect
The invention discloses a dendrobium officinale healthcare product with an auxiliary blood glucose reducing function. The dendrobium officinale healthcare product comprises the following raw materials in parts by weight: 35 to 45 parts of fresh dendrobium officinale bars, 1 to 2 parts of American ginseng, 2 to 4 parts of the root of kudzu vine, 2 to 4 parts of radix trichosanthis and 1 to 2 parts of fructus schizandrae. A preparation method for the dendrobium officinale healthcare product with the auxiliary blood glucose reducing function comprises the following steps: weighing the raw materials; processing the fresh dendrobium officinale bars; processing the American ginseng; performing alcohol extracting; performing water extracting; drying; batching. According to the dendrobium officinale healthcare product with the auxiliary blood glucose reducing function disclosed by the invention, the raw material formula is from clinical practice under the guidance of the theory of traditional Chinese medicine; the herbal medicines in a prescription are concise and are high in pertinency; the medicines in a raw material formula can nourish yin, benefit qi, clear heat and promote the secretion of saliva or body fluid; the active ingredients of the medicines are easy to absorb and are favorable to play efficacy; the medicinal materials in the raw material formula have the homology of medicine and food, and have no toxic or side effect.
Owner:广西弄峰山铁皮石斛科技有限公司
Panax Notoginseng microwave drying sterilization method
InactiveCN101336948AReduce heat lossImprove working conditionsFood processingFood preservationPANAX NOTOGINSENG ROOTMicrowave
The invention relates to a method for drying and sterilizing Panax notoginseng, which comprises the following steps of: (1) sorting Panax notoginseng raw material, washing, and draining; (2) turning on a microwave drying and sterilizing machine, laying the Panax notoginseng raw material on the conveyer belt of the microwave drying and sterilizing machine from the beginning end of the machine, controlling the laying thickness not more than 30 mm, conveying the Panax notoginseng raw material to a hermetic microwave radiation zone positioned in the middle section of the machine via the conveyer belt, and conducting drying and sterilizing, wherein the material feeding port is arranged in a common operation zone and the conveying speed of the conveyer belt is 0.3-5 m / min; and (3) conveying the dried and sterilized Panax notoginseng raw material out of the machine from the tail end arranged in a clean operation zone of the machine via the conveyer belt, collecting, bagging, and packaging. The inventive method adopts microwave drying and sterilization; and has the advantages of high heating speed, uniform heating, low drying temperature (lower than 80 DEG C), short drying time, and effectively saved energy consumption (by over 2 / 3).
Owner:云南特安呐制药股份有限公司
Preparation process of blood activating capsule
ActiveCN101773571AQuality improvementEasy to takeNervous disorderCapsule deliveryCitrus aurantium extractCyclodextrin
The invention discloses a preparation process of a blood activating capsule, which comprises the following steps that: first, angelica and citrus aurantium extract the fat-soluble effective ingredient groups through a supercritical CO2 extraction process, the fat-soluble effective ingredient groups are included by Beta-cyclodextrin to prepare inclusion volatile oil, herb residue and astragalus, peach kernel, safflower and other six Chinese medicines are decocted, the filtrate is concentrated into clear cream A, ethanol is added in so that the ethanol content is 65 percent, supernatant is taken out and the ethanol is recovered, and clear cream B is prepared by concentration; and finally clear cream C is purified by macroporous resin enrichment, the clear cream C is heated, sterilized, dried by microwave to prepare dry cream, and the dry cream is mixed with rhizoma ligustici wallichii fine powder and the included volatile oil and starch, boiled and granulated, and the capsule is filled. The preparation process of the blood activating capsule has the advantages that the blood activating capsule prepared has safe and reliable therapeutic effect and stable and controllable quality, abandons the ineffective and harmful ingredients in the Chinese medicine raw material, improves the medicine purity and reduces the dose of a patient.
Owner:西安大唐制药集团有限公司
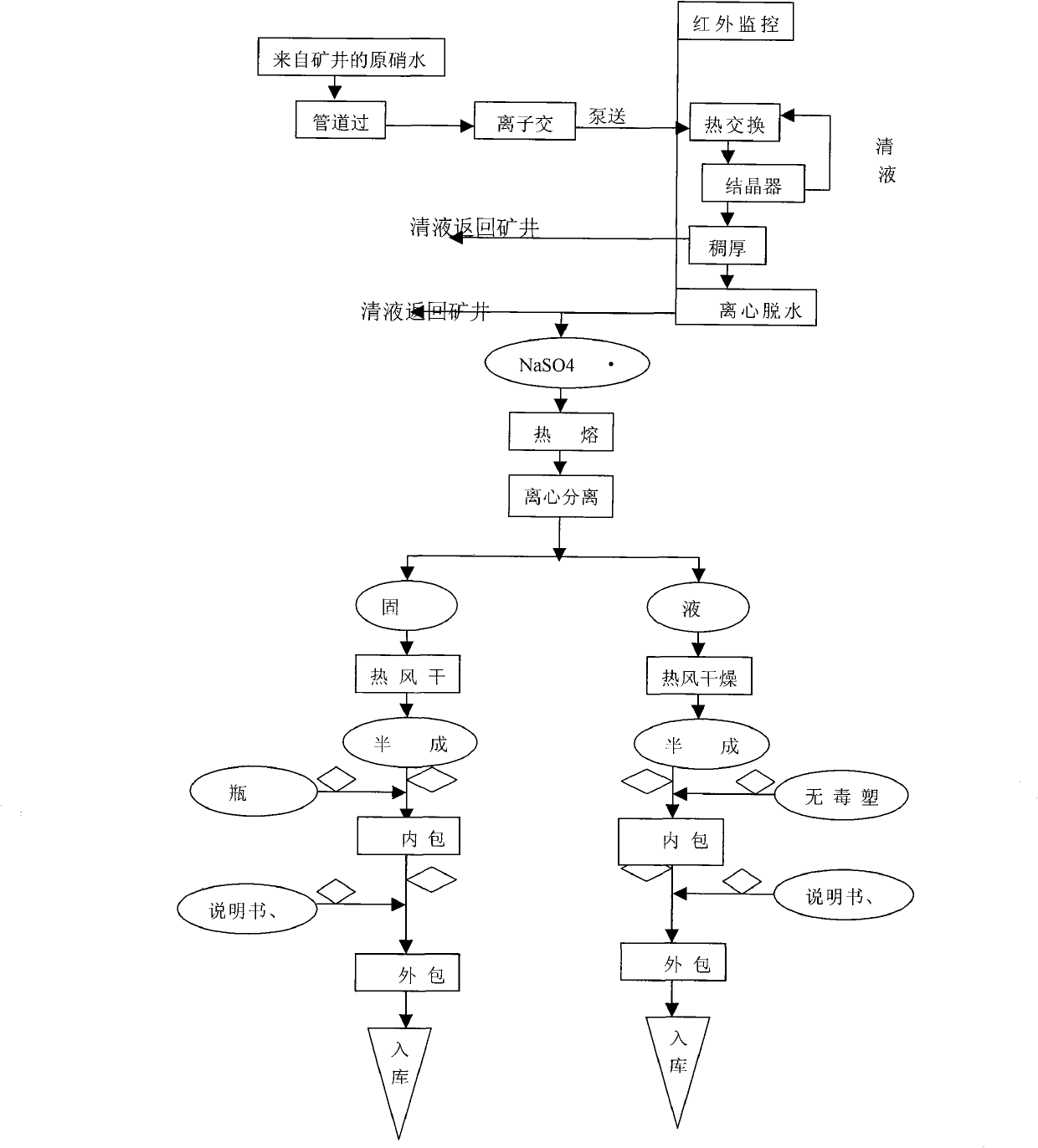
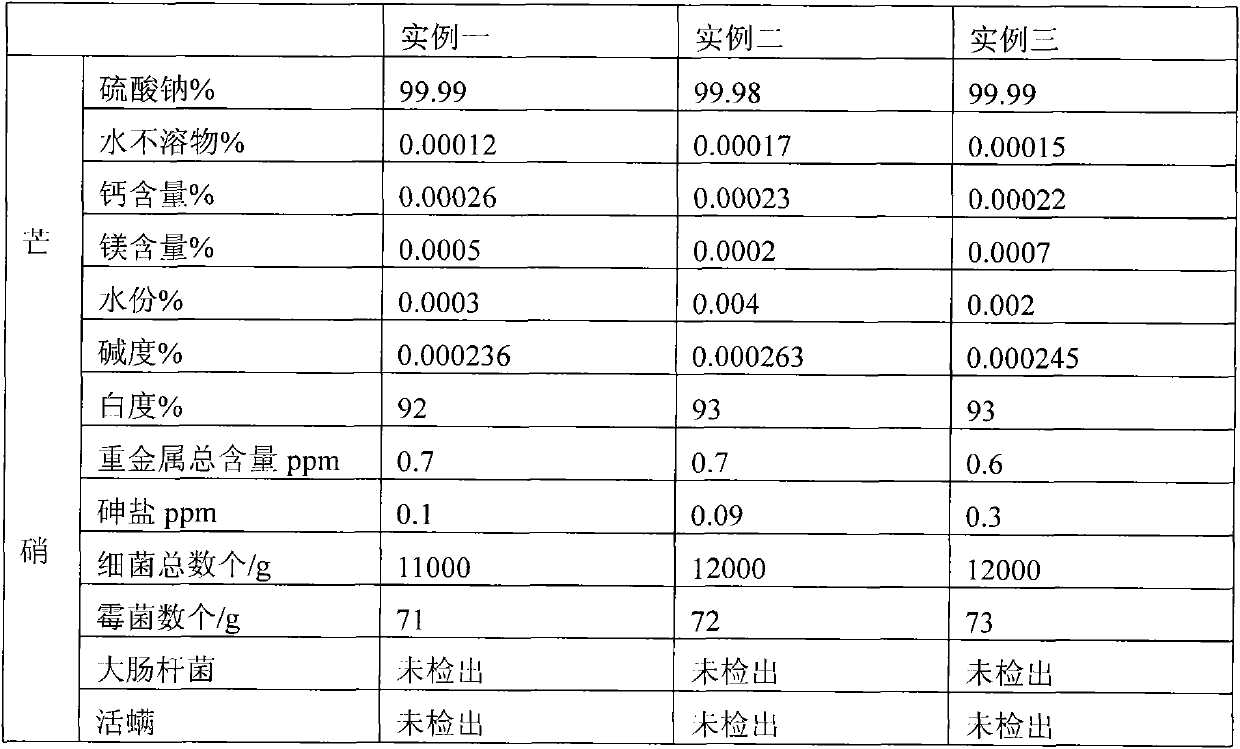
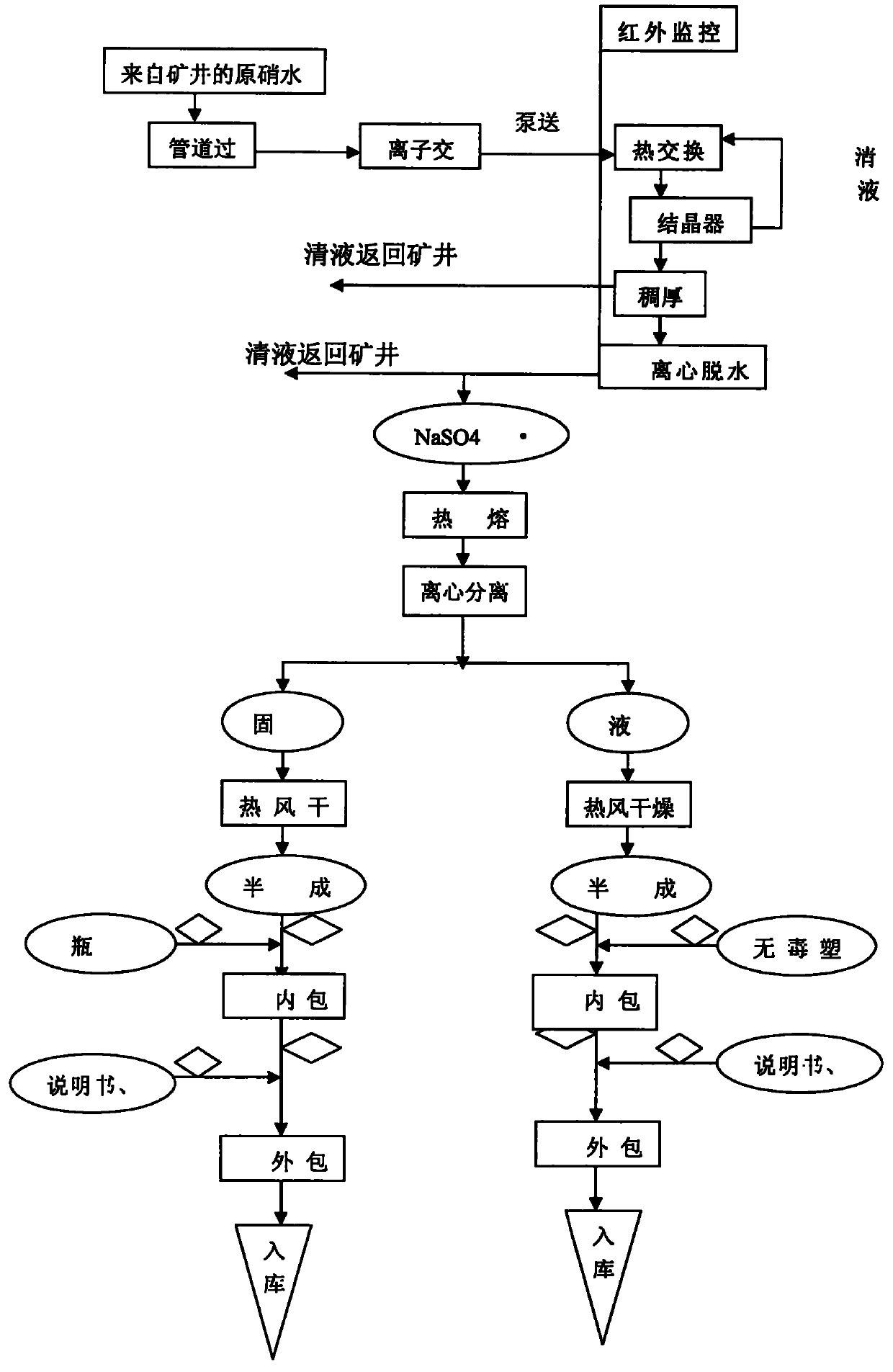
Technique for manufacturing anhydrous medicinal mirabilite
ActiveCN101948123AMonitor drynessMonitor cleanlinessAlkali metal sulfite/sulfate purificationIon exchangeEngineering
The invention relates to a technique for manufacturing anhydrous medicinal mirabilite. The method comprises the following steps: under the monitoring of an on-line infrared computer automatic monitoring system and the regulation and control of a porous plate liquid balance flowmeter, when the indoor air cleanliness of a production shop achieves a certain index, removing mechanical impurities from original nitrate solution (transported underground) with a certain concentration through a pipeline filter, purifying by ion exchange to obtain fine nitrate solution with low content of metallic ion impurities, inputting the fine nitrate solution into a continuous crystallization system, carrying out continuous solid-liquid separation on the crystallized crystal slurry through a strap type centrifugal machine to obtain medicinal mirabilite crystals, melting the medicinal mirabilite crystals in a steam heating or electric heating mode, carrying out solid-liquid separation, respectively drying solid and liquid with hot air to obtain the anhydrous medicinal mirabilite, and finally, carrying out aseptic packaging by using an automatic packaging system. On the premise of ensuring high product purity and conformity to GMP requirements, the invention has more ideal unit productivity.
Owner:四川省川眉药业有限公司
Airtight dust-proof granulation, drying and granule arranging integrated pharmaceutical device
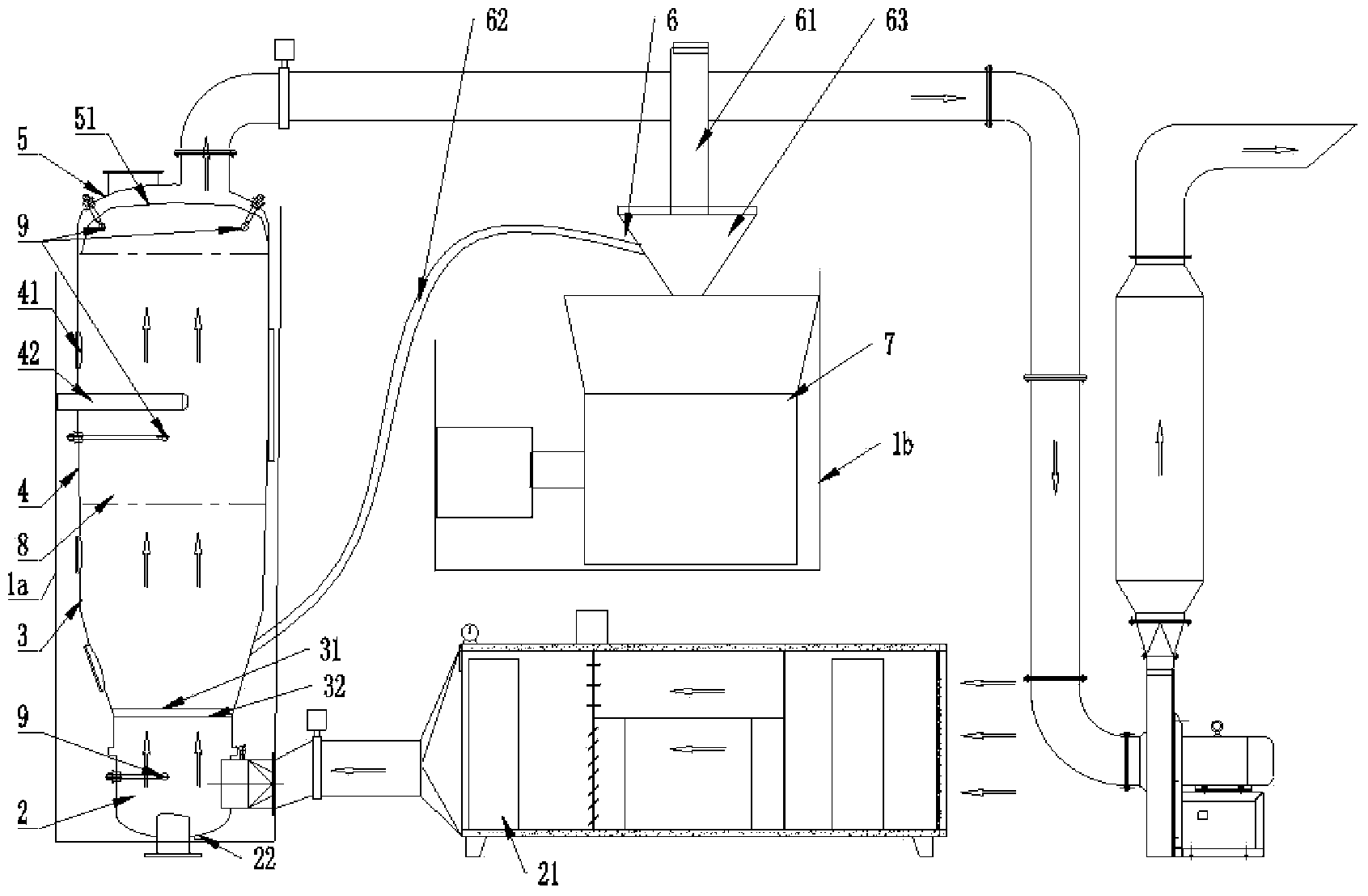
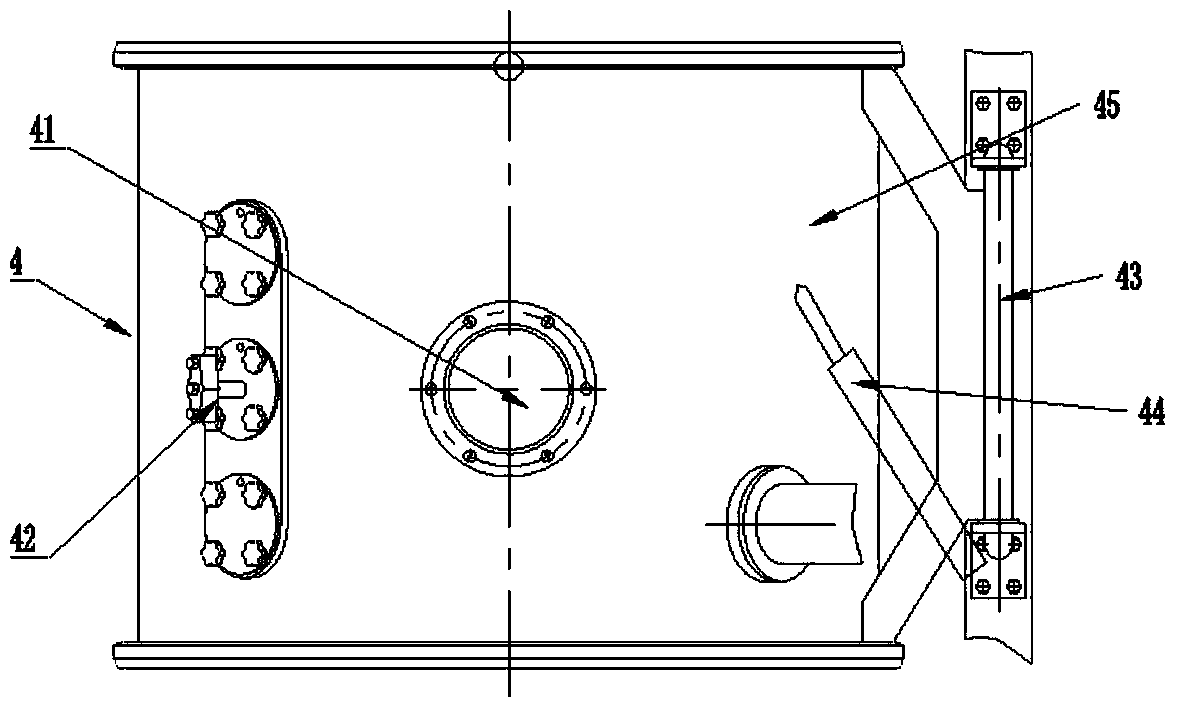
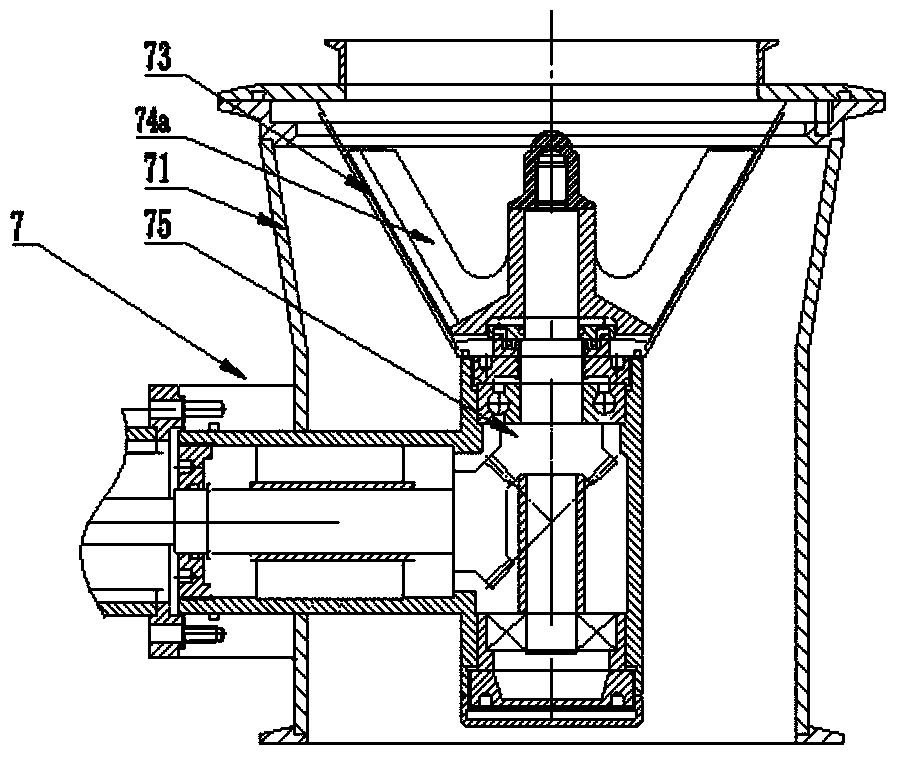
ActiveCN103505371AEffective controlReduce frequent cloggingPharmaceutical product form changeTowerAutomation
The invention discloses an airtight dust-proof granulation, drying and granule arranging integrated pharmaceutical device which is simple and reasonable in structure, airtight and free of pollution in the whole process and high in automation degree. The airtight dust-proof granulation, drying and granule arranging integrated pharmaceutical device comprises a material drying boiling cavity, a granule arranging system and a corresponding control device. The material drying boiling cavity is a tower type integrated drying boiling cavity which comprises an air inlet system, a granulation material bucket component, a middle bucket body component and an air exhausting dust removal system. A material conveying system is arranged between the granule arranging system and the tower type integrated drying boiling cavity, and the material conveying system comprises a conveying channel between the tower type integrated drying boiling cavity and the granule arranging system.
Owner:YICHUN WANSHEN PHARMA MACHINERY
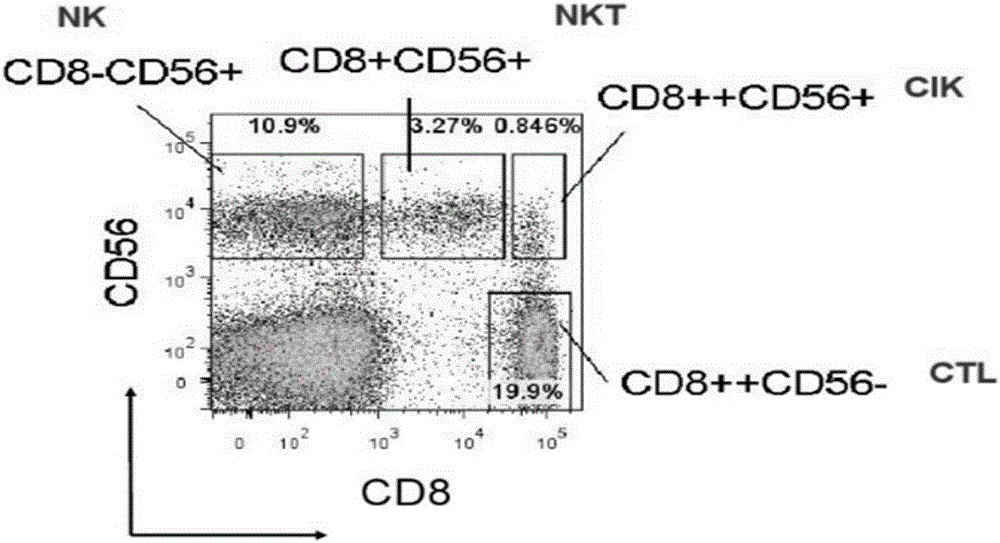
Method for preparing efficient clinical-level CD 56<+> group immune cell
InactiveCN106554942AIncrease the number ofComply with GMP requirementsMammal material medical ingredientsBlood/immune system cellsRabbit anti-human thymocyte immunoglobulinCD16
The invention provides a method for preparing efficient clinical-level CD 56<+> group immune cell. The method comprises the following steps: a) separating single karyocyte from peripheral blood or umbilical cord blood; b) inoculating the single karyocyte in the step a) to an anti-CD16 antibody-coated culture dish; c) adding rabbit-anti-human thymic cell immune globulin, Lifein and animal origin-free cytokine in the culture dish for inducing and stimulating the single karyocyte inoculated in the step b); d) supplementing the culture dish and the animal origin-free cytokine according to the cells counting results every 2-3 days; and e) obtaining the CD 56<+> group immune cell. The method can reduce the cost, accords with the requirement of clinical-level preparation, and has good killing activity in vitro and vivo.
Owner:JILIN TUO HUA BIOTECH
Pharmaceutical composition of Silybin and preparation method thereof
ActiveCN100594898CHigh yieldSolve the problem of large-scale industrial productionOrganic active ingredientsDigestive systemPhospholipid complexDrug product
The invention provides a silybin and phosphatidy lcholine compound which is prepared into capsules with high dissolving degree. The invention also provides a process for preparing the silybin and phosphatidy lcholine compound and its capsule medicament, the preparing process is characterized by simple manufacturing method, high yield and fitting for mass production.
Owner:天津天士力圣特制药有限公司
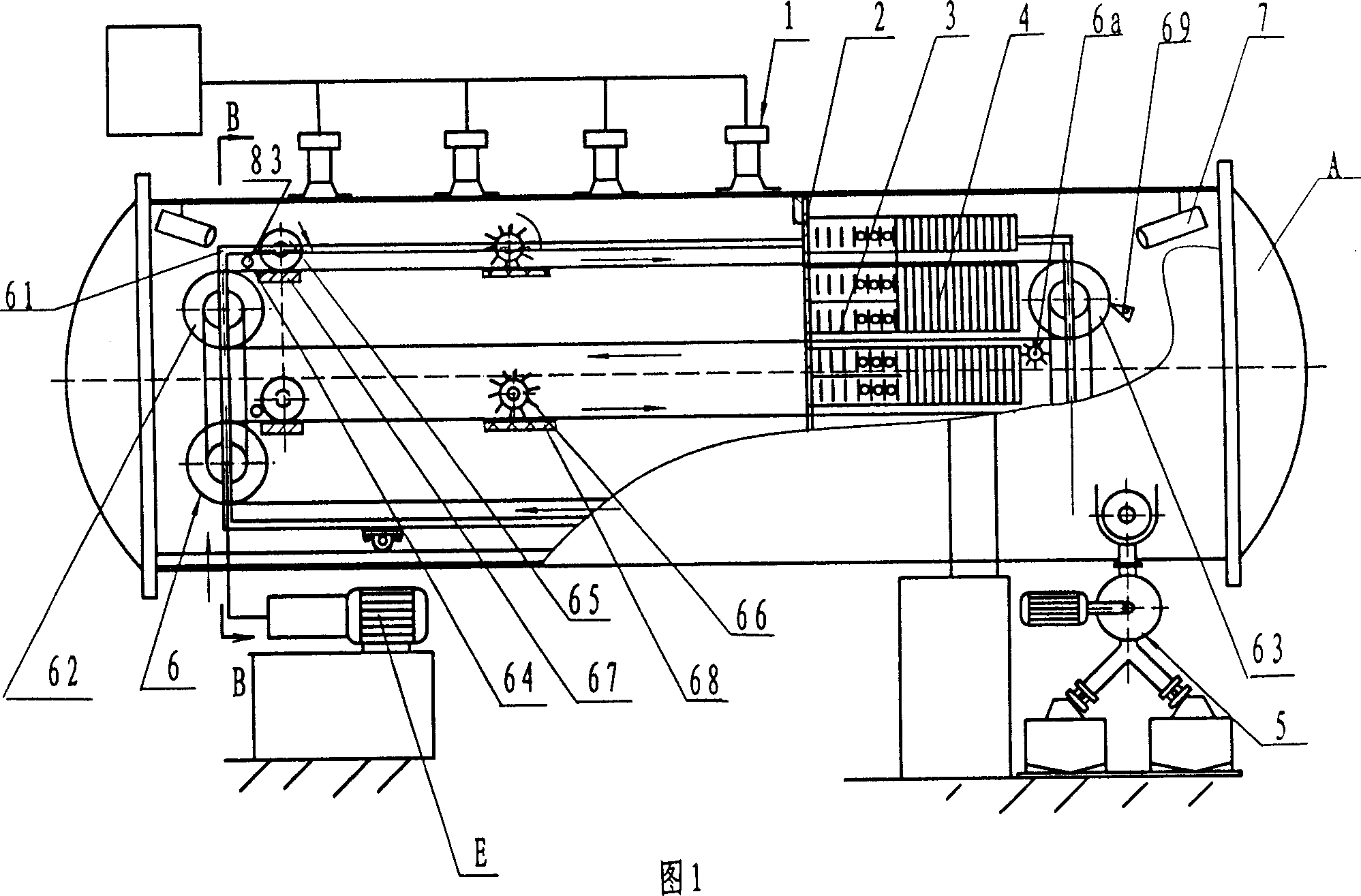
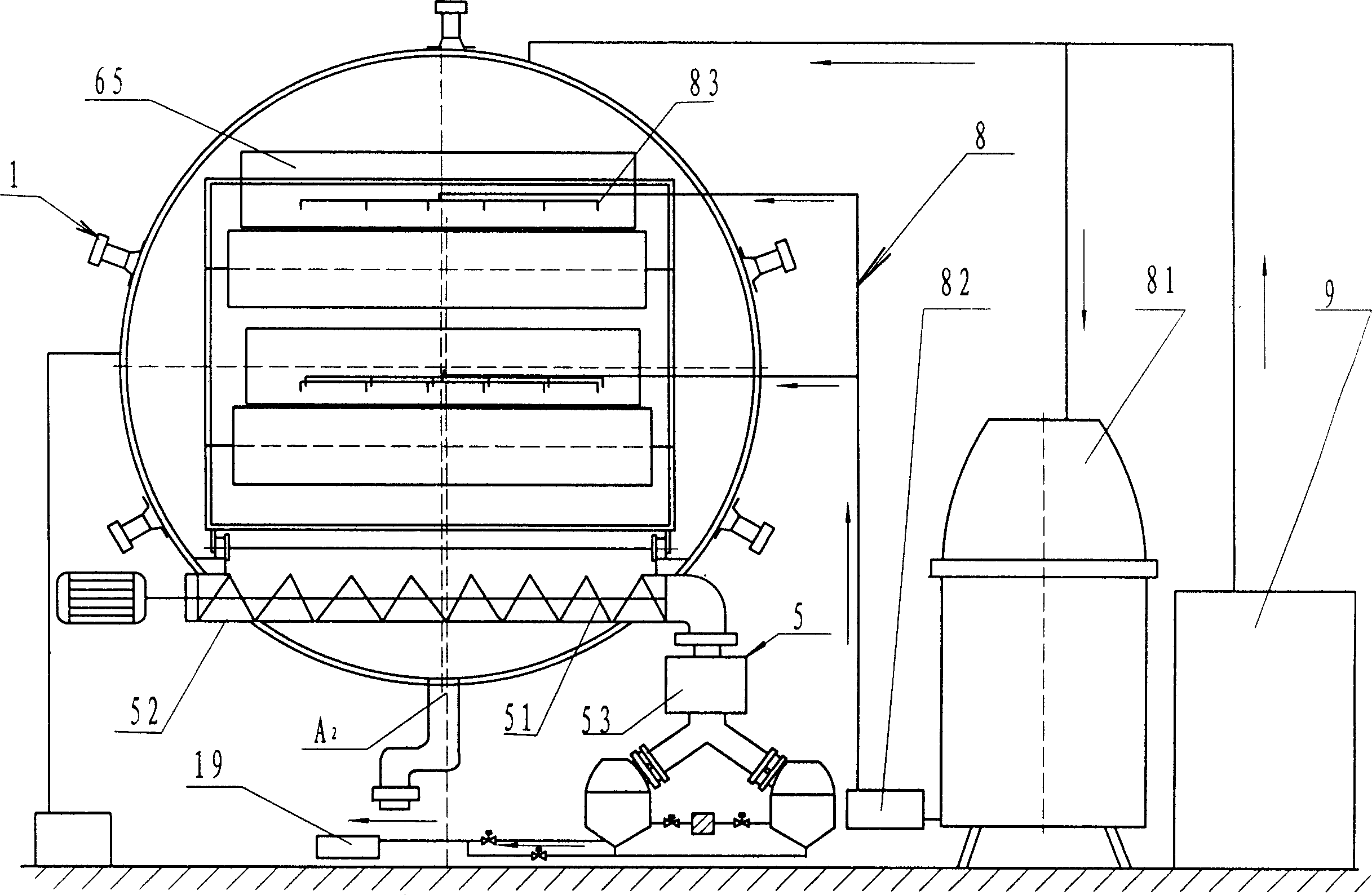
Safe and energy-saving microwave vacuum continuous automatic drier
InactiveCN1908561AImprove efficiencySlow heatingDrying solid materials with heatHearth type furnacesMetallic materialsToughness
The present invention mainly relates to a microwave drier, especially a microwave vacuum drier. A safe and energy-saving microwave vacuum continuous automatic drier includes a tank (A), a hoisting system (8), a vacuum system (9), a microwave heating system (1) on the tank (A), characterized by including a delivery device (6) inside the tank (A), a discharging device (5) below the delivery device (6), the materials in the tank (A) are non-metallic materials. On the basis of ensuring the strength and the toughness, the striking a light and discharging are avoided in the vacuum state when metal is heated in the microwave. The invention adopts the way of microwave radiation heating, utilizing the characters of microwave high efficiency, easy to control, antisepsis, sterilization, fast heating, even heating, fast dehydration, preserving to reduce the power waster and advance the efficiency.
Owner:TIANSHUI HUAYUAN PHARMA EQUIP TECH
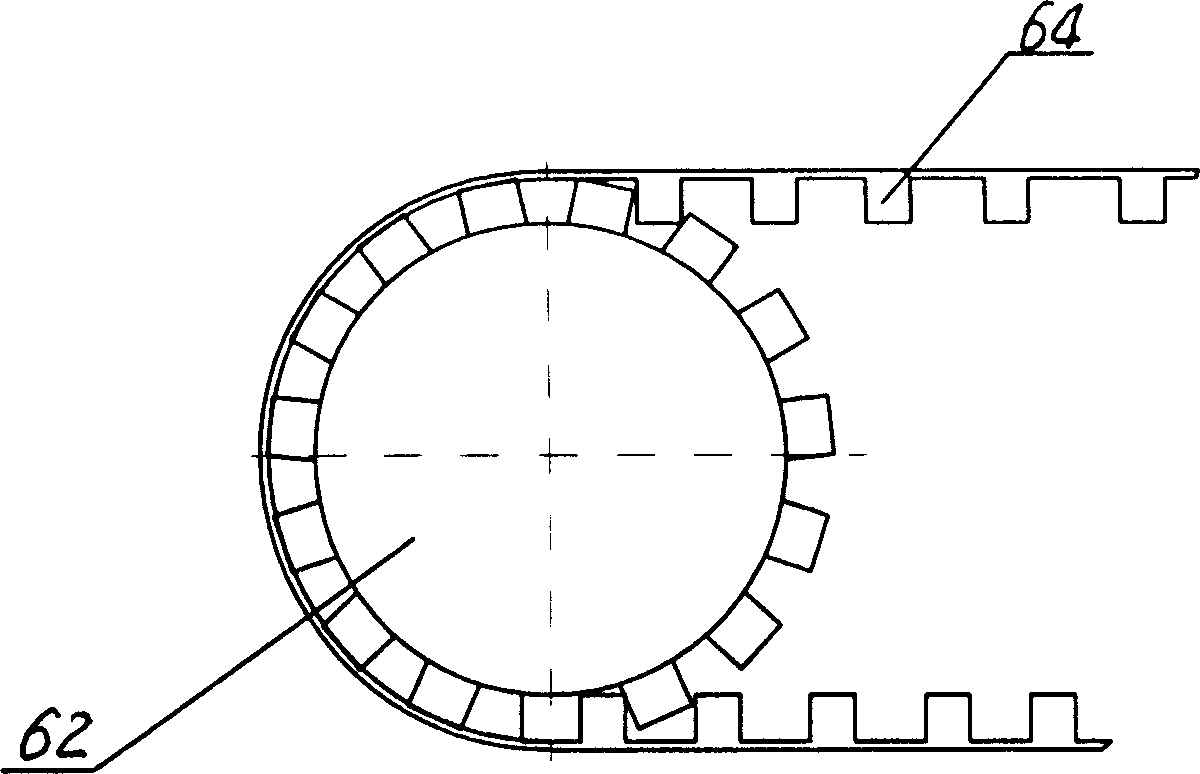
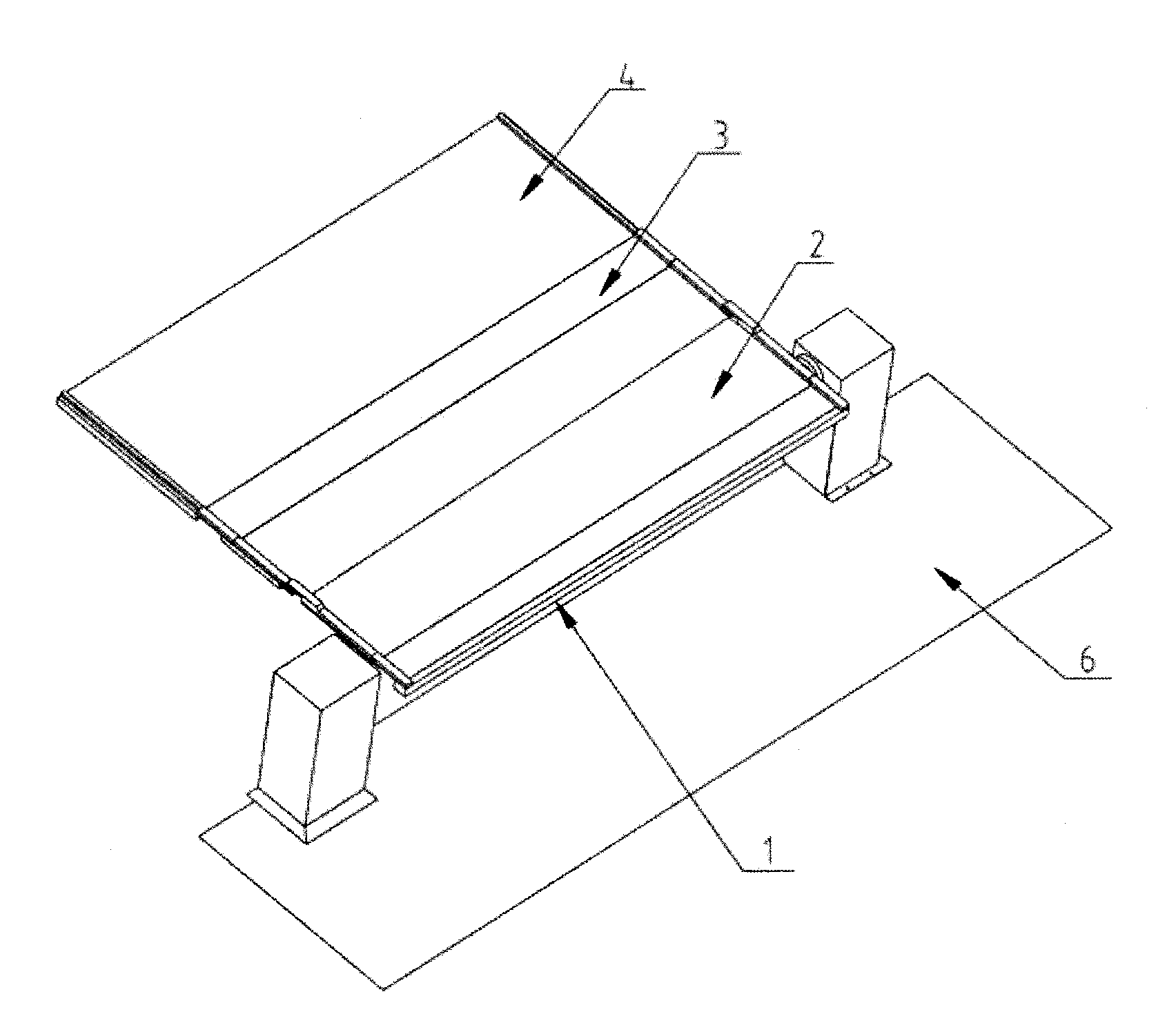
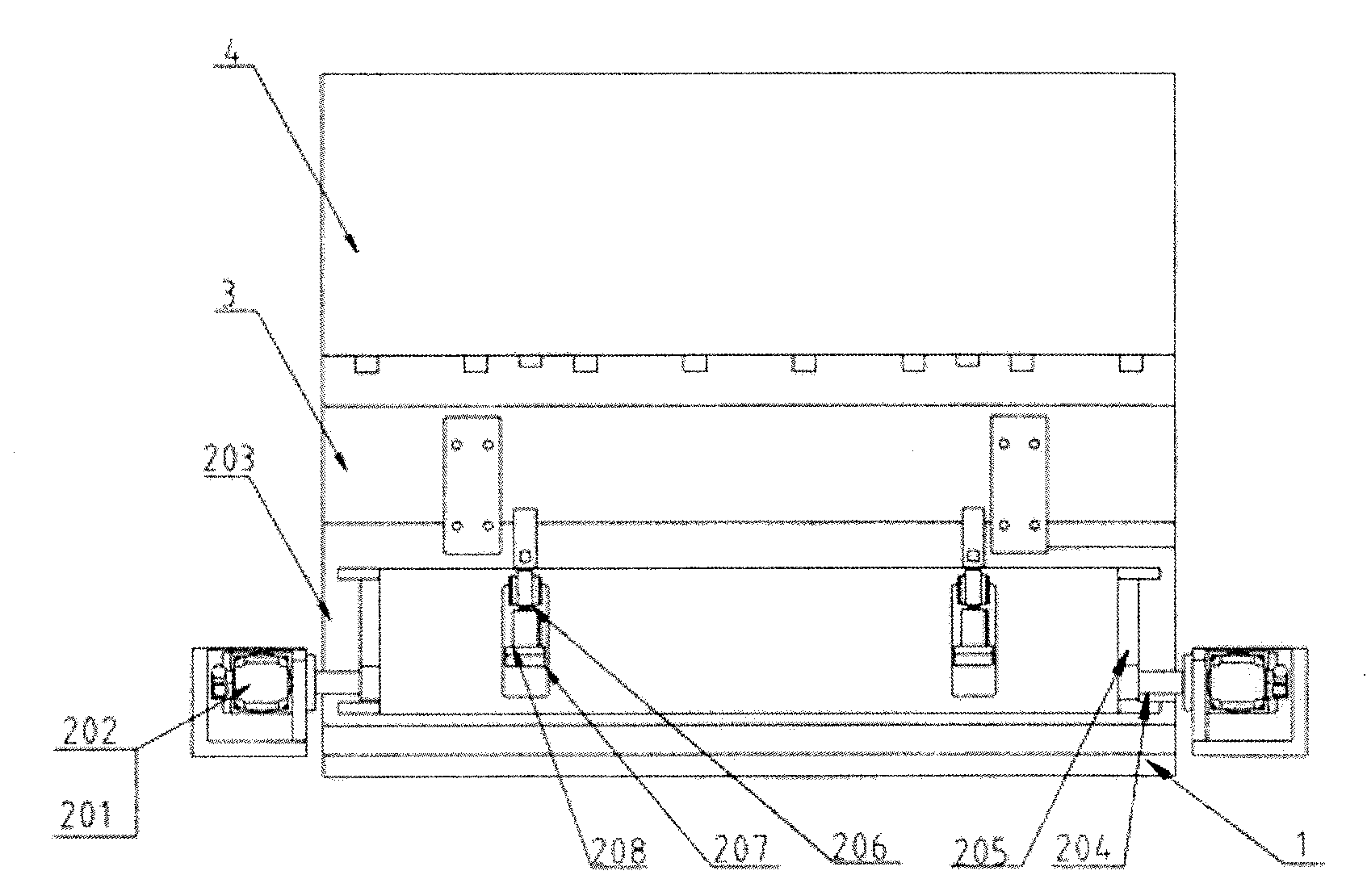
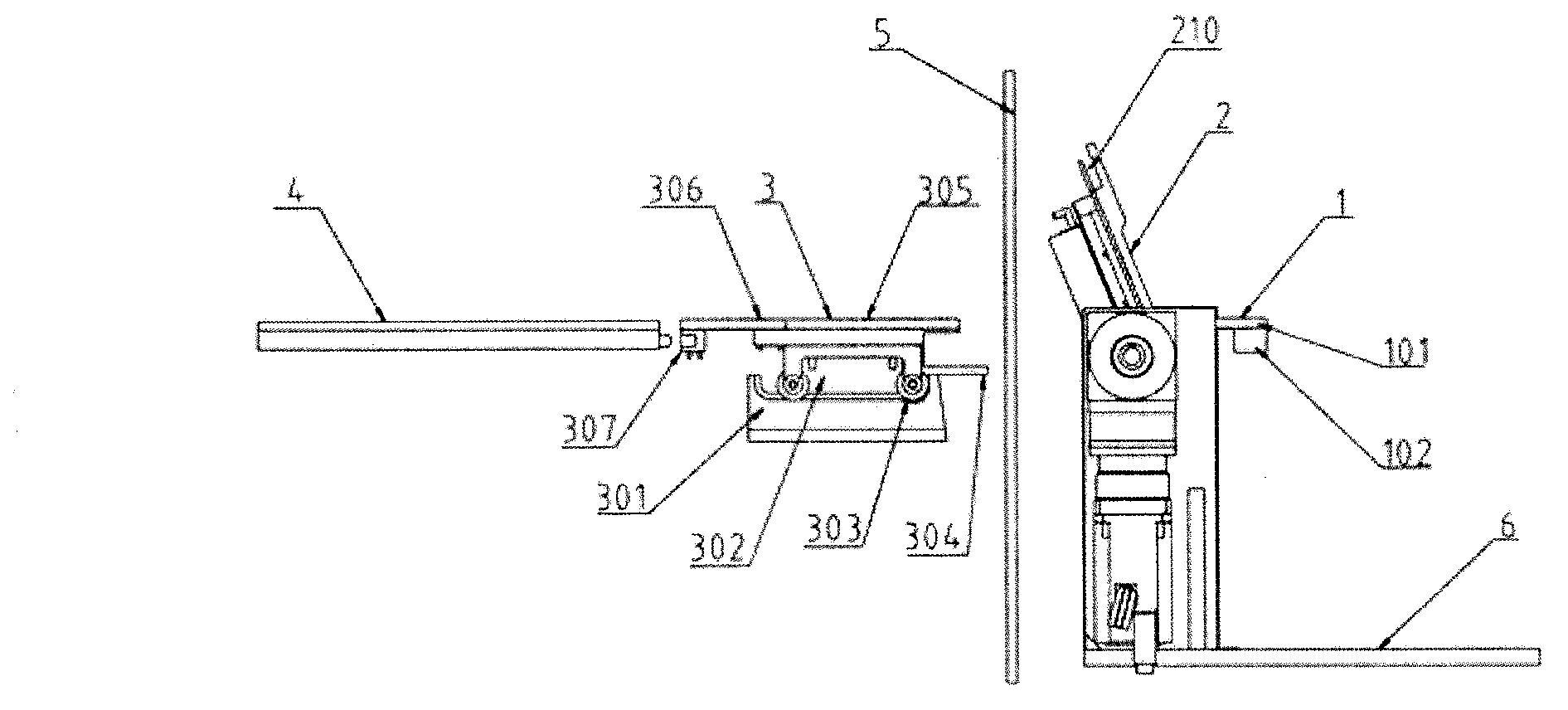
Three-section type butt-joint device
InactiveCN103322790AButt firmHigh-precision dockingDrying solid materialsTranslation systemButt joint
The invention discloses a three-section type butt-joint device which is characterized by comprising a base plate. A butt-joint turning-over system is arranged on the base plate, a butt-joint fixing system is arranged on one side of the butt-joint turning-over system, and a butt-joint translation system is arranged on the other side of the butt-joint turning-over system. The three-section type butt-joint device guarantees sterility of a medicine factory and production process continuity, is stable in butt joint with a plate layer and can achieve fast and high-precision butt joint.
Owner:SHANGHAI TOFFLON SCI & TECH CO LTD
Process for preparing injection liquid of brain protein hydrolyzate
InactiveCN1129451CEasy to operateIncrease equipment investmentHydrolysed protein ingredientsUnknown materialsMillipore FiltersPepsin
A process for preparing the liquid injection of brain protein hydrolyzate includes mixing water with pig's brain, homogenizing, filtering, hydrolyzing with pepsin and then pancreatin, regulating pH value, laying aside, filter, adding water to regulate pH value to be neutral, filtering with millipore filter, superfiltering with ultrafiltering membrane, heating, cooling, and ultrafiltering.
Owner:HAINAN STAR PHARM CO LTD
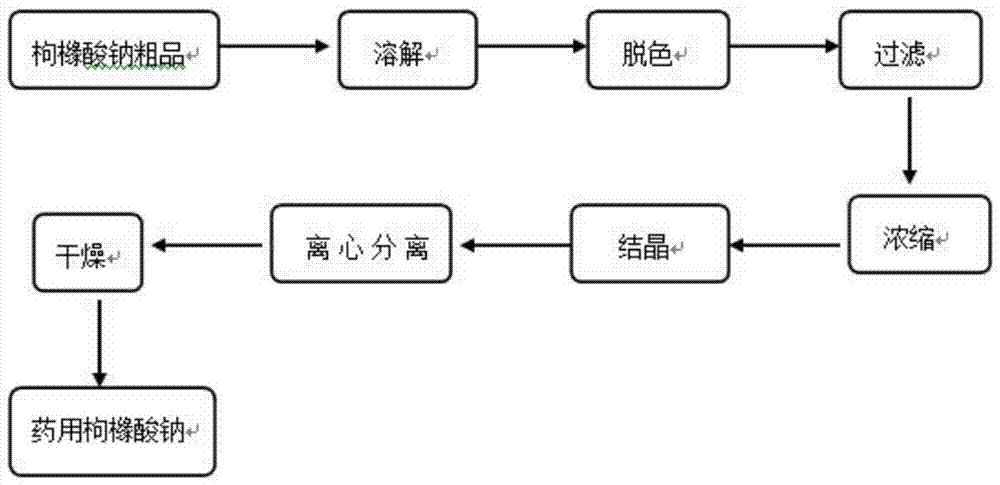
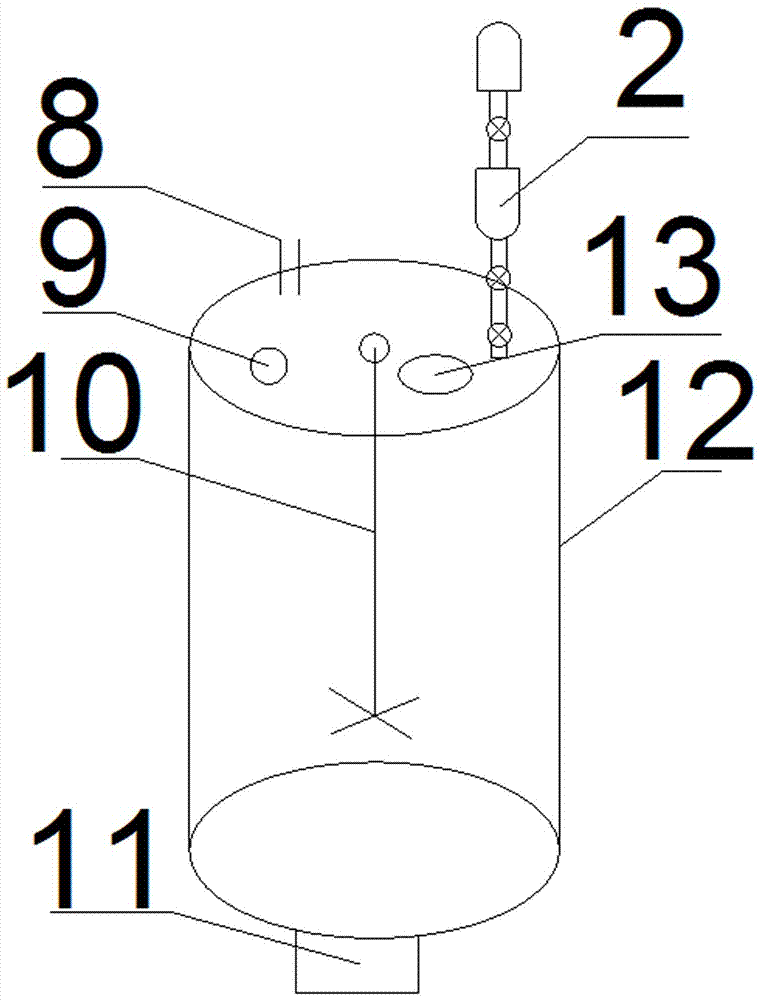
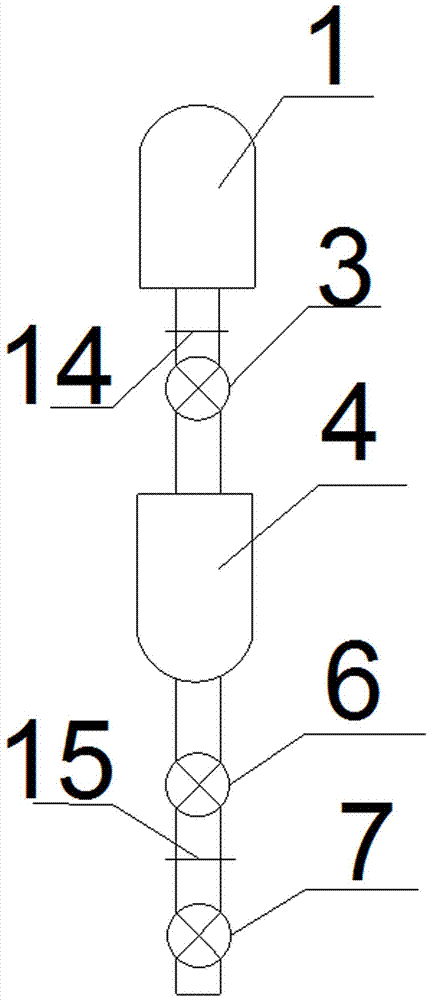
Granularity-controllable sodium citrate refining process and implementing device
InactiveCN107188798APurification process conditions are mildLow costCarboxylic compound separation/purificationPhysical chemistrySodium citrate
The invention discloses a granularity-controllable sodium citrate refining process and an implementing device. The process comprises the following steps: preparing an anhydrous sodium citrate crude product solution, performing decoloring treatment so as to obtain fine filtrate, concentrating the fine filtrate so as to obtain a vicious liquid, performing cooling crystallization so as to obtain wet sodium citrate granules, performing centrifugal treatment on the wet sodium citrate granules, and performing vibration fluidizing drying, thereby obtaining pure sodium citrate crystal granules. By adopting the process, preparation steps and purification processes are optimized, particularly key process parameters of concentration and crystallization are optimized, the purity of the prepared sodium citrate is greater than 99.9% and is as high as 99.99%, the yield of the sodium citrate is greater than 69%, and the sodium citrate is controllable in particle size range, simple in production process, low in cost, simple and feasible to operate, environmentally friendly and safe.
Owner:湖南新绿方药业有限公司
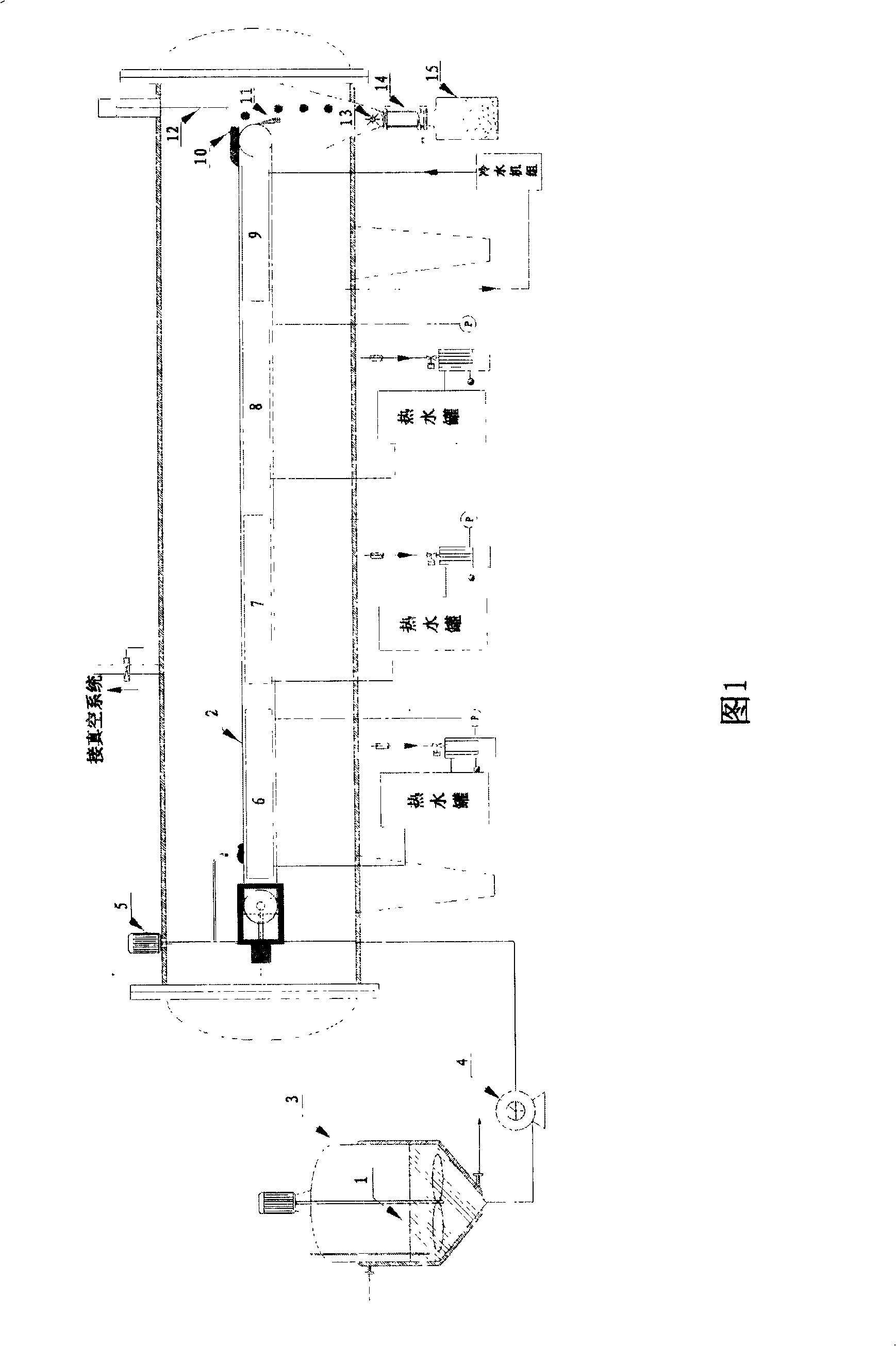
Chinese medicine kudzu root extract new drying technique method
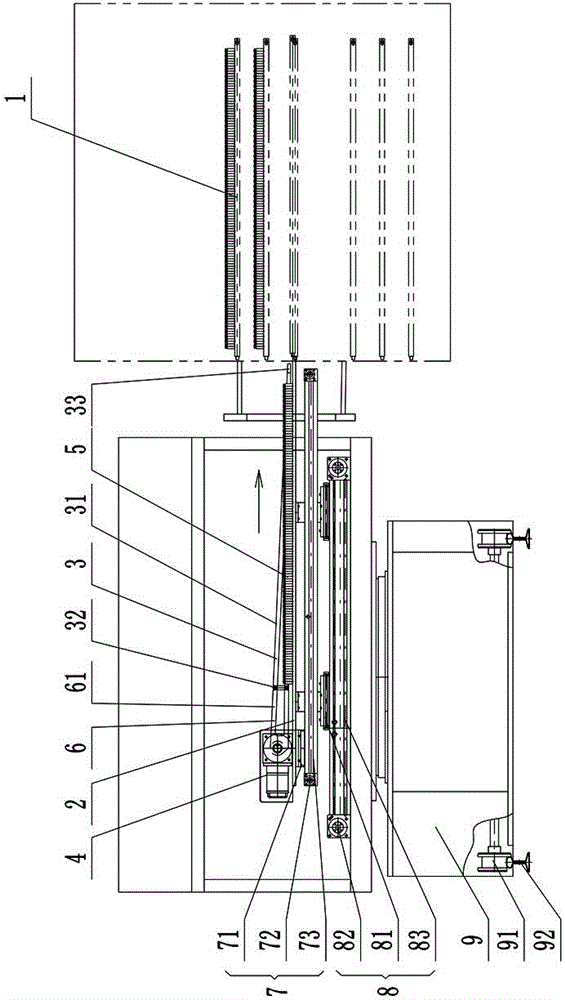
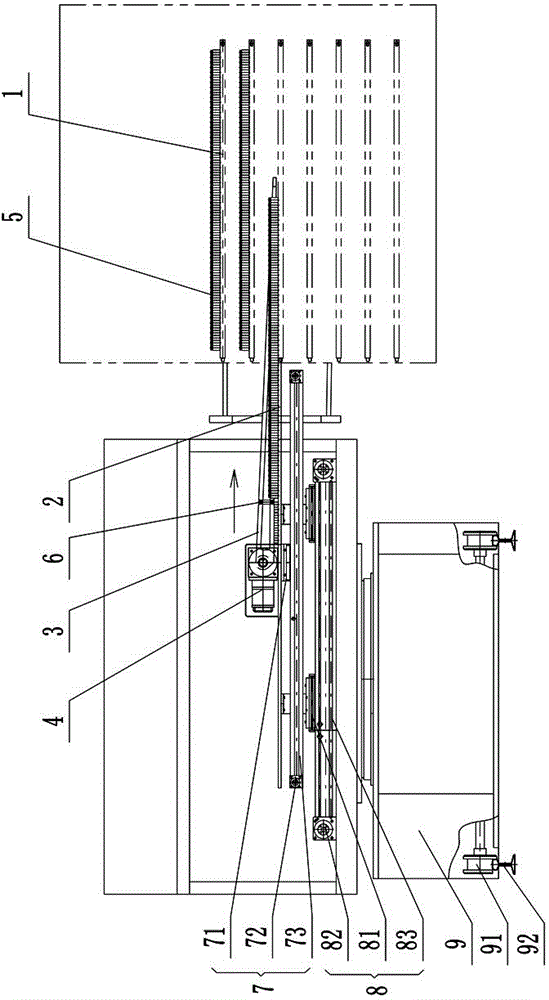
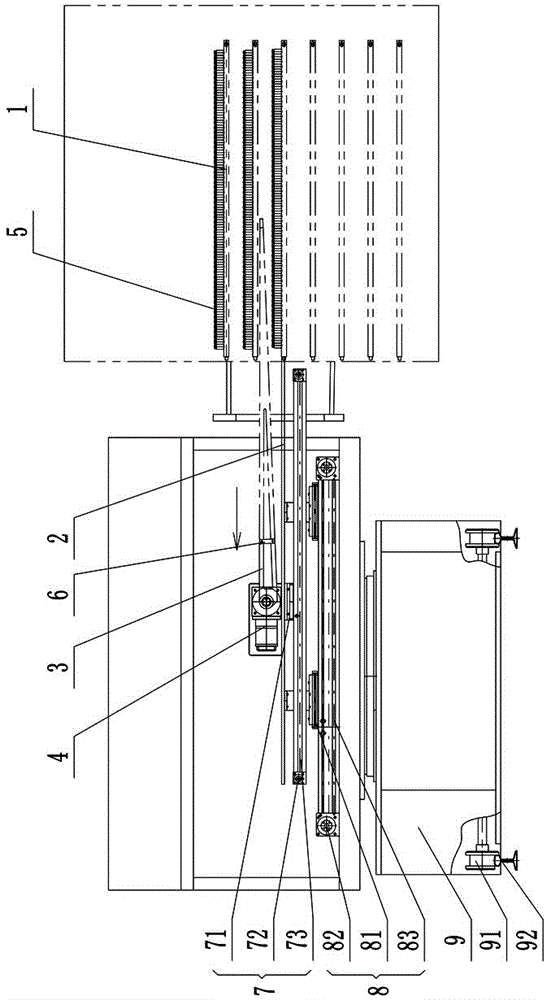
ActiveCN101259157AReduce moisture contentMaintain physical propertiesDrying solid materials with heatAntipyreticSolubilityAutomatic control
The invention discloses a new drying process for kudzu root extract of Chinese medicine. Kudzu root extract (1) is preheated in a charging stock tank (3) and evenly coated on a conveyer belt (2) by a screw charging pump (4) through a stock distributing gear (5). Under the vacuum condition, the kudzu root extract (1) moves and passes through heating sections (6) (7) (8) in sequence as along with the conveyer belt (2), and goes through a cooling section (9) at last, forming a porous, loose and cake-like dry substance. By adopting the method, the kudzu root is dried step by step under the vacuum conditions; the whole drying process is mild and short in time. The obtained dry substance is a porous and loose medium, which has the advantages of light color, low water content and good solubility. Operating environment is totally enclosed to prevent people from the cross infection between people and material, which meets GMP requirement. Compared with the drying method of a vacuum baking oven operated by hand, the whole drying process realizes continuously full automatic control and greatly reduces labor intensity and improves production efficiency.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
Automatic feeding and discharging device and feeding and discharging method
ActiveCN104444161AAvoid situations that create debrisReduce frictionMechanical conveyorsBiochemical engineeringBottle
The invention discloses an automatic feeding and discharging device and a feeding and discharging method. The device comprises a bottle collecting bedplate and a bottle moving assembly, the bottle moving assembly comprises a bottle catching frame located on the bottle collecting bedplate, the bottle catching frame is composed of side frame plates, a rear frame plate and a front frame plate, the rear frame plate is connected with a rotating driving part, and the bottle catching frame rotates so that the front frame plate can be switched between a bottle blocking state and a bottle avoiding state. The feeding and discharging method comprises the following steps that feeding is conducted, wherein the bottle collecting bedplate is in butt joint with a target bottle storage plate, and the bottle catching frame of the bottle moving assembly catches bottles on the bottle collecting bedplate and pushes the bottles to the target bottle storage plate; after feeding is finished, the bottle catching frame is rotated so that the front frame plate can be in the bottle avoiding state, and the bottle catching frame retreats out of the target bottle storage plate; discharging is conducted, wherein the bottle collecting bedplate is in butt joint with the target bottle storage plate, the bottle catching frame of the bottle moving assembly moves to the target bottle storage plate, the bottle catching frame is rotated so that the front frame plate can be in the bottle blocking state, and the bottle catching frame catches the bottles on the target bottle storage plate to the bottle collecting bedplate. The automatic feeding and discharging device and the feeding and discharging method have the advantages that operation is simple and convenient, the structure is simple and GMP requirements are met.
Owner:TRUKING TECH LTD
Preparation method of rehmannia leaf total glycoside extract dry product
InactiveCN101703613AGentle drying processShort drying timeUrinary disorderPlant ingredientsWater contentPulverizer
The invention relates to a preparation method of a rehmannia leaf total glycoside extract dry product, which comprises the steps: firstly, the rehmannia leaf total glycoside extract is preheated in a charging stock tank and then uniformly coated on a running conveyor at the speed of 2-8m / h by a screw charging pump according to the 1-2.5kg / h charging speed through a distributing device; in the vacuum condition, the rehmannia leaf total glycoside moves and passes through a first heating section and a second heating section in sequence, and finally a cooling section to form porous and loosened caky dry product; when the dry product reaches the tail end of the device, the dry product is shaved off from the conveyor by a stripper, cut off by a cutting device and falls into a stranding cage pulverizer; and then the crushed dry product enters a product collecting tank by a discharging device. The obtained dry product is characterized by having low water content and good solubility, and being porous and loosened. Compared with a common vacuum oven drying method, the quality of the product obtained by the method is considerably increased and is beneficial to the follow-up preparation technology of the rehmannia leaf.
Owner:SICHUAN MEDCO PHARML
Ointment for treating coronary disease and its production process
InactiveCN1813912AImprove ischemic stateGood curative effectOrganic active ingredientsAerosol deliveryCoronary artery diseaseMyrrh
The present invention discloses a Guanxin adhesive plaster and its production process. It is made up by using the Chinese medicinal materials of salvia root, ligusticum root, Chinese angelica root, carthamus flower, myrrh, clove, frankincense, dalbergia wood, camphor, xylene musk, menthanol, diphenhydramine hydrochloride, borneol and medical carrier rubber plaster matrix through a certain preparation process. Besides, said invention also provides the concrete steps of said preparation process.
Owner:李秀花
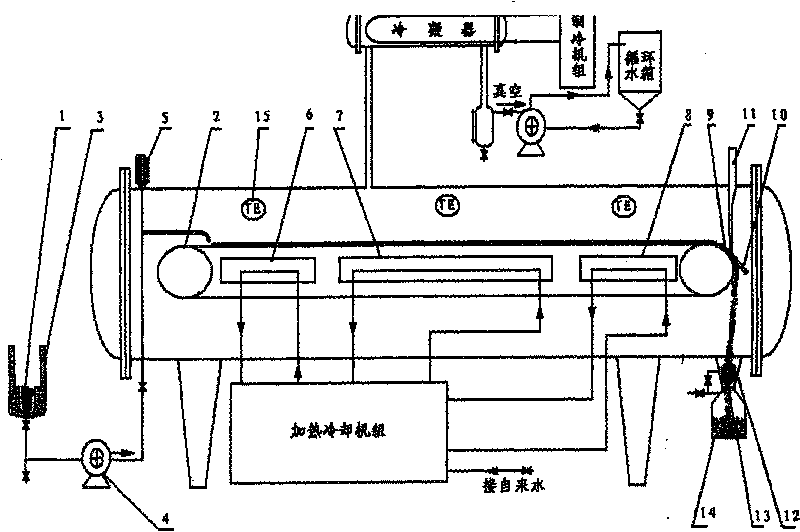
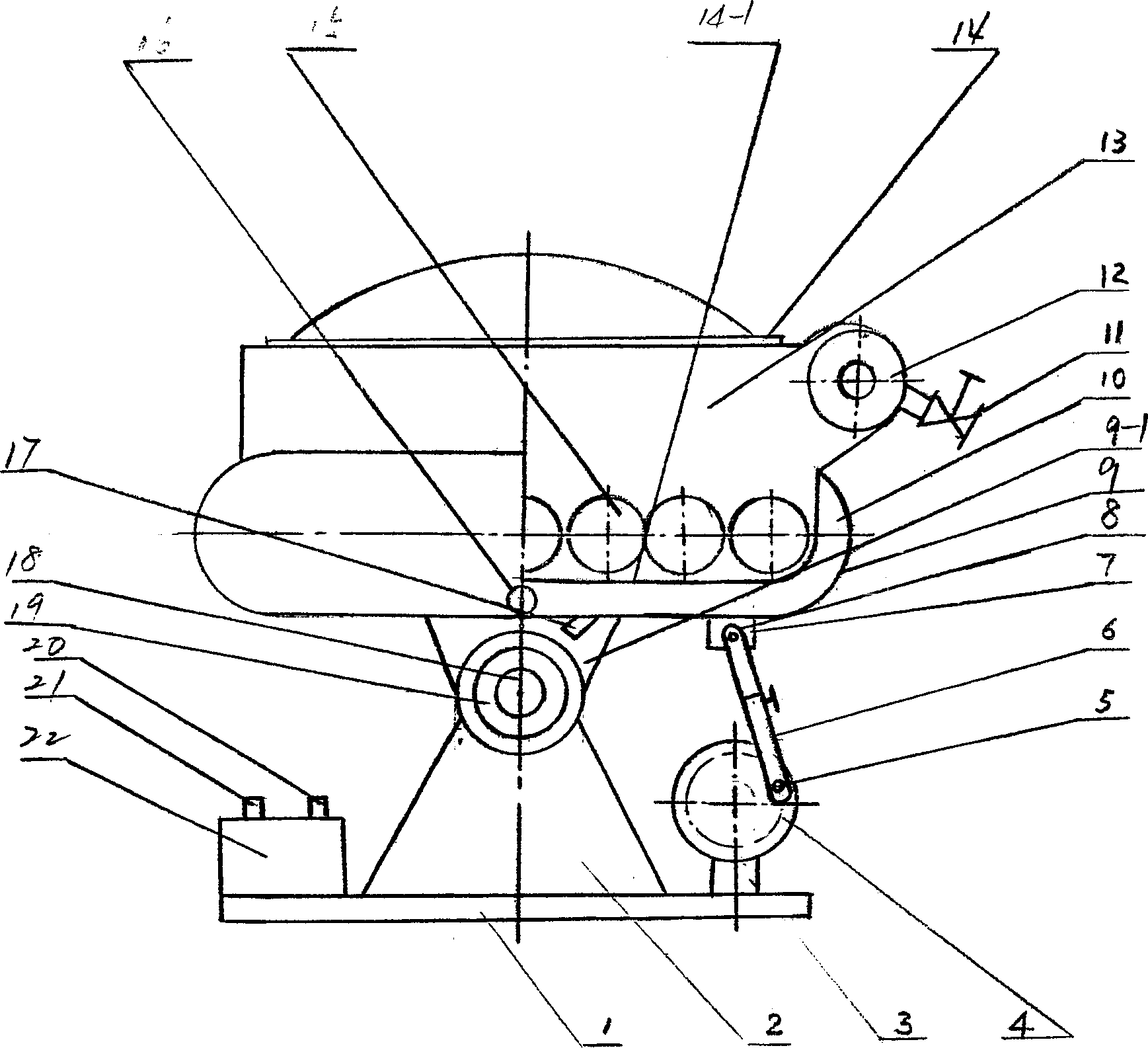
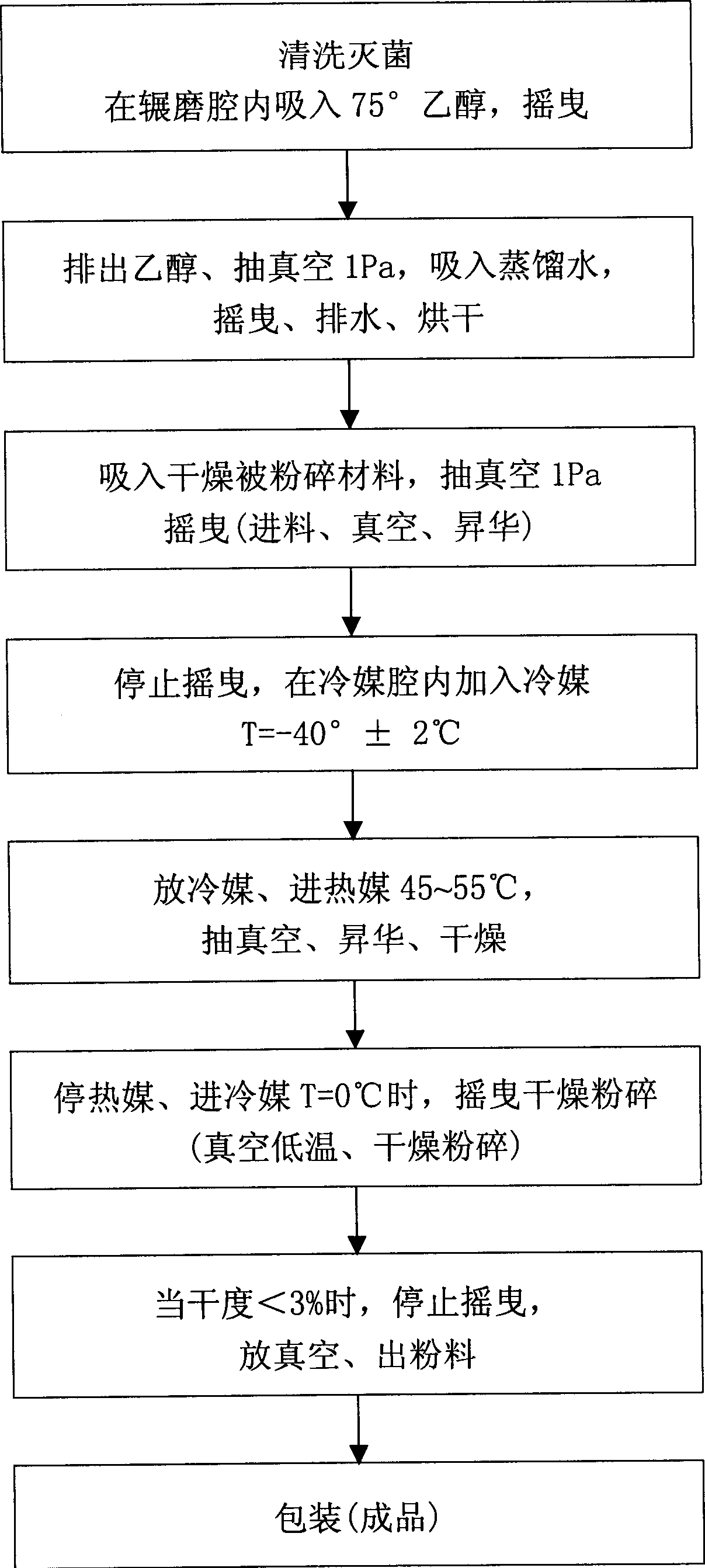
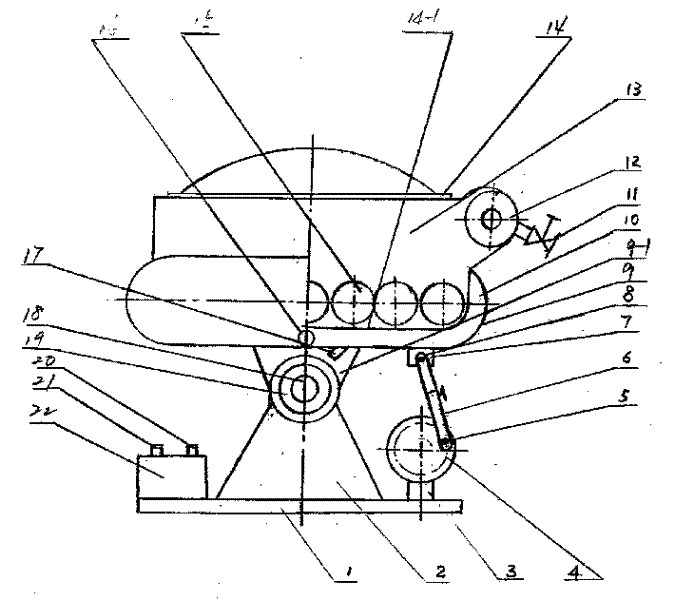
Method for designing high-vacuum freeze, sublimation, drying and pulverization integrated machine and its equipment
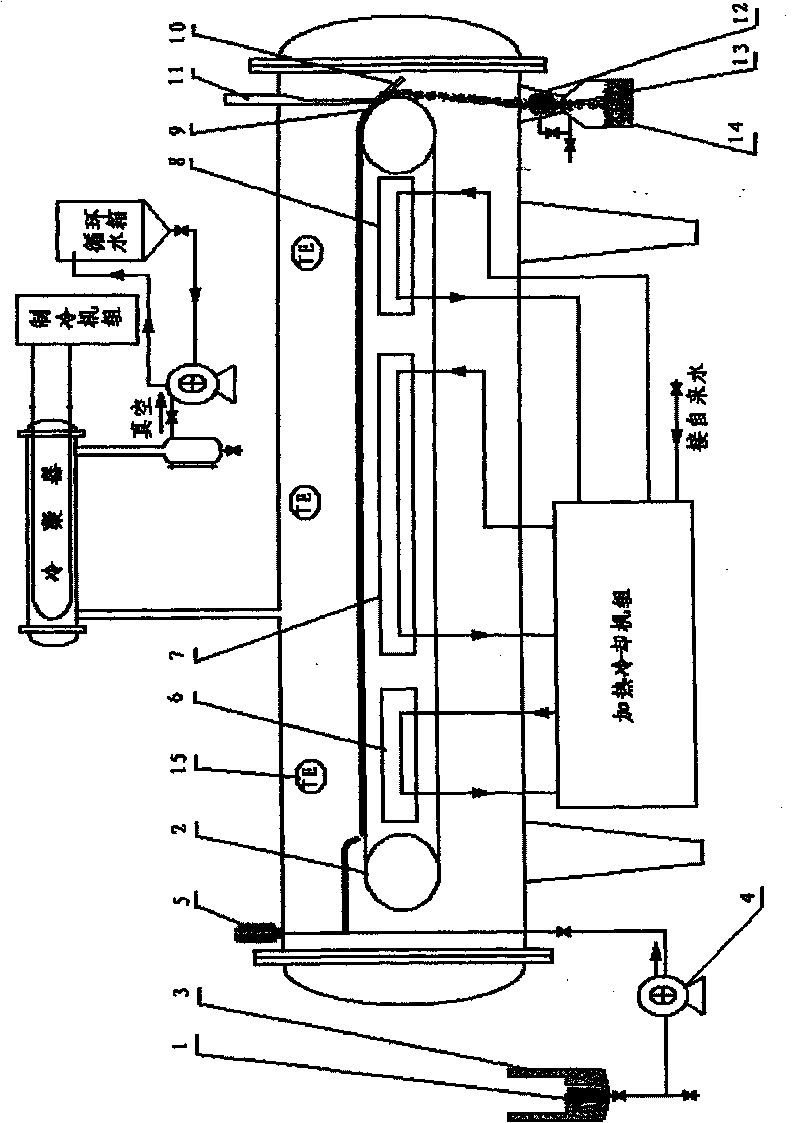
InactiveCN1444000AImprove qualityComply with GMP requirementsDrying solid materials without heatPulp and paper industryThermite
The present invention relates to a pulverizing machine, in particular, it is a design method of high-vacuum freezing, sublimation, drying and pulverizing integrated machine and its equipment. It adopts vacuum freezing design, and its equipment has a grinding cavity body, said cavity body is separated into grinding cavity and refrigerant cavity by means of "U"-shaped partition board, and the flour-discharging screw shaft is communicated with grinding cavity, and another end of the telescopic connecting bar is connected with a shake-pulling power device supported by its support frame, and on the grinding cavity the thermite inlet and outlet, refrigerant inlet and outlet and vacuum pump interface are set.
Owner:上海敏杰机械有限公司
An in-situ automatic cleaning device for a freeze dryer
ActiveCN102278872ASolve the problem of cleaning in placeControllable water volumeDrying solid materials without heatCleaning processes and apparatusFreeze-dryingSpray nozzle
The invention discloses an in-place automatic washing device for a freeze dryer. The in-place automatic washing device comprises a guide rail arranged in a freeze drying cabin, wherein a trolley base is arranged on the guide rail, washing comb supports which are arranged in a layering way are connected to the trolley base, a spray nozzle is arranged on each washing comb support, a water pipe coiling drum and an air pipe coiling drum are arranged on the trolley base and connected with coaxial coiling drum shafts, a water pipe and an air pipe are respectively arranged on the water pipe coiling drum and the air pipe coiling drum, an inlet of the water pipe and an inlet of the air pipe are respectively connected with a water pipe joint and an air pipe joint which are arranged on the lower end of the trolley base, an outlet of the water pipe and an outlet of the air pipe are respectively connected with a pneumatic valve through a rotating joint, and an outlet of the pneumatic valve is connected with the washing comb supports. According to the invention, the problem of in-place washing of the freeze drying cabin is solved, and reciprocated washing and blowdown can be automatically carried out according to actual requirements in terms of programs. The in-place automatic washing device has the characteristics of program control, adjustable water quantity, economy and high efficiency, simple structure, no need of manpower and space, stability and safety, reciprocated washing automaticity and no dead angle and second pollution and accords with GMP (Good Manufacturing Practice) requirements.
Owner:北京创思佳信息技术有限责任公司
Preparing method of fleabane extract dry matter
InactiveCN101015577AReduce moisture contentMaintain physical propertiesCardiovascular disorderPlant ingredientsErigeronEngineering
The invention discloses a porous Erigeron brevsicapus extract dry substance and its preparation method. The method comprises pre-heating Erigeron brevsicapus extract in feed tank, spreading uniformly on a convey belt with a distributor, delivering to pass through each heating regions and a cooling region sequentially to form porous, loosened and dried substance, scrapping the substance off the convey belt, cutting off with a cutting apparatus, pulverizing, and collecting. The whole process is performed under vacuum and mild conditions for a short period of time. The produced dried extract has porous and loosened texture, light color, low water content and good dissolubility. The inventive drying method helps to increase the product quality, as compared with the existing vacuum-oven drying method.
Owner:ZHEJIANG UNIV
Method for preparing pharmaceutics of hydrolysate of brain protein
ActiveCN1228082CTo achieve the separation effectNo pollution in the processPowder deliveryNervous disorderHydrolysateTyrosine
A hydrate of brain protein is prepared from pig's brain through adding purified water, homogenizing, heating, cooling, regulating pH=1.5-2.0, enzymolyzing, regulating pH=7.7-8.0, enzymolyzing, regulating pH=2.5-3.0 freezing, thawing, filter, regulating pH to become neutral, ultrafiltering, concentrating, sterilizing, adding amino acids, regulating peptide map, diluting, and steam sterilizing.
Owner:赛隆药业集团股份有限公司
Traditional Chinese medicine supersonic air jet crushing method
InactiveCN110404654AReduce energy consumptionConcentrated particle size distributionGrain treatmentsTraditional Chinese medicineFluidized bed
The invention discloses a traditional Chinese medicine supersonic air jet crushing method, and relates to the field of traditional Chinese medicine crushing. The traditional Chinese medicine supersonic air jet crushing method aims to solve the problem of how to safely crush existing flammable and explosive traditional Chinese medicines during crushing. According to the traditional Chinese medicinesupersonic air jet crushing method, a traditional Chinese medicine supersonic air jet crushing system is adopted to crush the traditional Chinese medicines, and comprises a nitrogen making machine, agas storage tank, a constant nitrogen supplementing electromagnetic valve, a nitrogen compressor, a freezing dryer, an oil remover, a pressure distributor, a closed feeding system, a crushing classifier, a cyclone collector, a bag-type dust remover, a pressure relief opening, a vacuum blow-off valve, a nitrogen content analyzer, a hammer crusher and an air-cooling type universal crusher. According to the traditional Chinese medicine supersonic air jet crushing method, on the basis of the design of a traditional fluidized bed type crusher, a self-flow-dividing classifying technology is combined, so that the crushing equipment adopting the nitrogen for protection is designed, and then safe crushing of the combustible and explosive traditional Chinese medicines is realized. The traditional Chinese medicine supersonic air jet crushing method is applied to the field of traditional Chinese medicine crushing.
Owner:龙晖药业有限公司
Andrographolide nanometer suspension agent
ActiveCN109568265ASimple prescriptionReduce dosageAntibacterial agentsOrganic active ingredientsChemistryNanometre
The invention provides an andrographolide nanometer suspension agent. The andrographolide nanometer suspension agent includes andrographolide particles with the effective average particle size less than 1000 nm and one or more anionic surface stabilizers. According to the nanometer suspension agent, the prescription is simple, the preparation process is simplified and controllable, industrialization is easy, the efficiency is high, preparation stability is good, and dilution resistance is achieved.
Owner:SHINEWAY PHARMA GRP LTD +1
Preparation method of diclofenac potassium sustained-release pellet capsule
ActiveCN101612140AStable blood concentrationSmall fluctuationOrganic active ingredientsAntipyreticSustained Release CapsuleDiclofenac Sodium
The invention relates to a preparation method of diclofenac potassium sustained-release pellet capsule. The preparation method sequentially comprises: preparing diclofenac potassium hormone pill by an extrusion-spheronization mothod, packing an isolation layer, packing a sustained-release layer and encapsulating the capsule, extruding the capsule into a stick on the condition that the set frequency of an extruder is 10-100Hz, and spheronizing the capsule for 3-30min on the condition that the frequency of a spheronization machine is 20-150Hz. In the invention, flying dust does not occur, the pollution is little and the production process meets the requirement of GMP; after the prepared diclofenac potassium sustained-release capsule is taken orally, the plasma concentration of the drug is gentle with small fluctuation and the capsule has good sustained-release effect.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Full-function osmotic pump controlled-release tablet laser-beam drilling machine
ActiveCN102699539ALow failure rateComply with GMP requirementsSortingLaser beam welding apparatusFailure rateColor discrimination
The invention relates to a full-function osmotic pump controlled-release tablet laser-beam drilling machine. The device comprises a two-orbit tablet vibration disk feeder, a double-layer flat belt type conveyer, a color discrimination sensor, a drilling positioning sensor, a laser, a high-velocity scanning vibration mirror, a computer and a vision detection removing system. By adopting the structural design of the two-orbit tablet vibration disk feeder and the double-layer flat belt type conveyer, controlled-release tablets are increased from original 5-10 tablets to 15-30 tablets per second through, the failure rate of the continuous operation of the controlled-release tablets is lowered, and the defects of poor adaptive capacity and easy material buckling of a rotating disk type mechanical sequencing structure are overcome; all operation is carried out in a stable and continuous operating state, and therefore, accurate positioning and good drilling consistency are achieved; by adopting the vision detection removing system, accurate removal is achieved, and therefore, the defects of incomplete removal and excess removal which are generated by adopting a pneumatic device are prevented; and the vision detection removing system detects the controlled-release tablets one by one and discriminates and removes imporous and polyporous tablets and tablets with unqualified apertures and pore sites, and therefore, the requirements of the GMP (Good Manufacturing Practice) of industrial production are met.
Owner:天津工业自动化仪表研究所有限公司
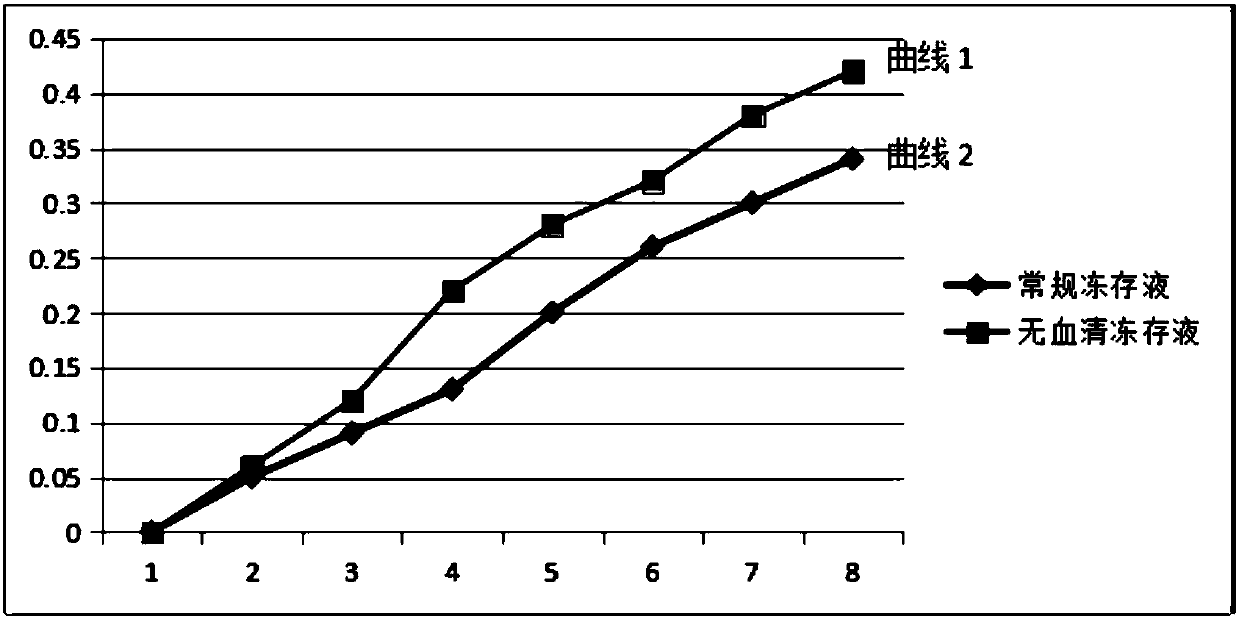
Serum-free cryopreservation liquid for neural stem cells
InactiveCN107743955APromote proliferationImprove featuresDead animal preservationAdditive ingredientAdenosine
The invention provides cryopreservation liquid for human neural stem cells, belongs to the technical field of stem cell cultures, and is a stem cell cryopreservation liquid composition suitable for clinic application. Various ingredients are used for optimizing the cryopreservation conditions; the cryopreservation liquid is prepared from the following ingredients in percentage by volume: 65 to 75percent of serum-free culture media of human neural stem cells, 3 to 5.5 percent of traditional Chinese medicine of rhizoma gastrodiae sourced monomeric compound solution, 9 to 12 percent of dimethylsulfoxide, 3 to 5.5 percent of adenosine, 2.5 to 5 percent of human neural stem cell sourced exosome solution and 2.5 to 5 percent of human neural stem cell sourced oligopeptide and polypeptide compound solution. After the cryopreservation of the human neural stem cells in the cryopreservation liquid, the recruitment rate reaches 85 percent or higher. No fetal calf serum is contained in the cryopreservation liquid; the exogenous unclear ingredient and pathogenicity pollution is prevented; the quality stability and safety and the standardized production of the stem cell treatment product are effectively ensured; the foundation is laid for the clinic treatment.
Owner:XUZHOU NORMAL UNIVERSITY
Fresh herbs and/or volatile medical material full ingredient preparation and storing and taking medicine
The invention relates to fresh herbs and / or volatile medical material full ingredient preparation and a storing and taking medicine. The preparing and storing method comprises the following steps of collecting or purchasing fresh herbs and / or volatile medical materials; performing water-retention sealed packing after removing impurities and additives; removing the impurities, cleaning and draining off; sterilizing by using ozone; freezing quickly and storing at low temperature; preparing lyophilized fresh herbs and / or volatile medical materials and collecting aboriginal liquid and hydrotrope; pulverizing the lyophilized fresh herbs and / or volatile medical materials at low temperature to prepare fresh herbs and / or volatile medical material lyophilized micronized powder; and respectively filling the fresh herbs and / or volatile medical material lyophilized micronized powder and the aboriginal liquid and storing the fresh herbs and / or volatile medical material lyophilized micronized powder and the aboriginal liquid in a vacuum environment or a nitrogen-filling environment. By the preparing and storing method of fresh herbs and / or volatile medical material full ingredients, problems that in a fresh herbs and / or volatile medical material collecting, storing, processing and taking method, effective ingredients are volatilized and lost, and go mouldy and bad, herbal properties are degraded, absorptivity is low, artificial additives and pollution are caused are solved.
Owner:齐生伟
Dendrobium candidum ganoderma lucidum capsule
ActiveCN102772672BNo pollution in the processNarrow particle size distributionMetabolism disorderAntinoxious agentsDendrobium candidumHigh absorption
The invention discloses a dendrobium candidum ganoderma lucidum capsule, and relates to a traditional Chinese medicine capsule, in particular to a dendrobium candidum ganoderma lucidum capsule free from influence of air humidity and having high absorption and high bioavailability and a preparation method thereof. The dendrobium candidum ganoderma lucidum capsule disclosed by the invention is characterized by being prepared by the four steps of processing of dendrobium candidum, ultrafine grinding of dendrobium candidum, ultrafine grinding of ganoderma lucidum and mixing and capsule filling. In the dendrobium candidum ganoderma lucidum capsule disclosed by the invention, the ultrafine powder of dendrobium candidum and the ultrafine powder of ganoderma lucidum are mixed and packed in a capsule form, the influence of air humidity can be avoided, and the human body absorption and the bioavailability are high; and moreover, the dendrobium candidum ganoderma lucidum capsule has the functions of dendrobium candidum and ganoderma lucidum, and has a better taking effect.
Owner:XISHUANGBANNA ZENGLIANG BIOTECH
Recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen, production method of hepatitis B surface antigen, hepatitis B vaccine and production method of hepatitis B vaccine
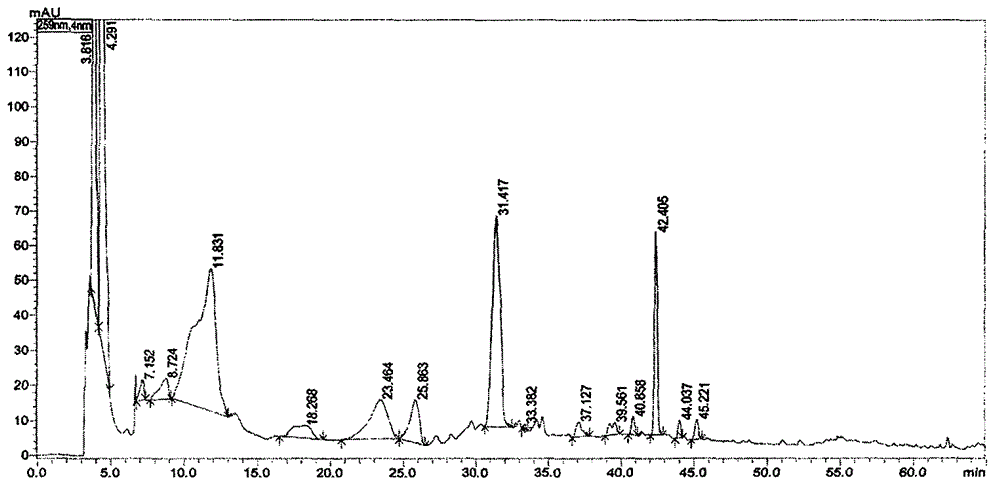
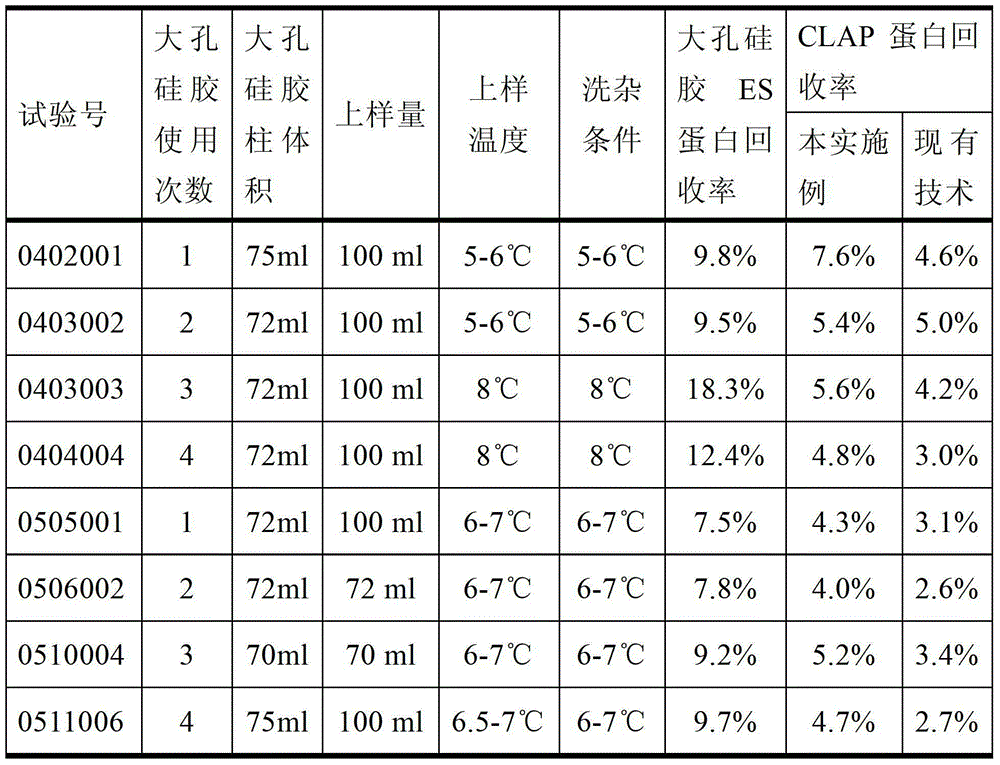
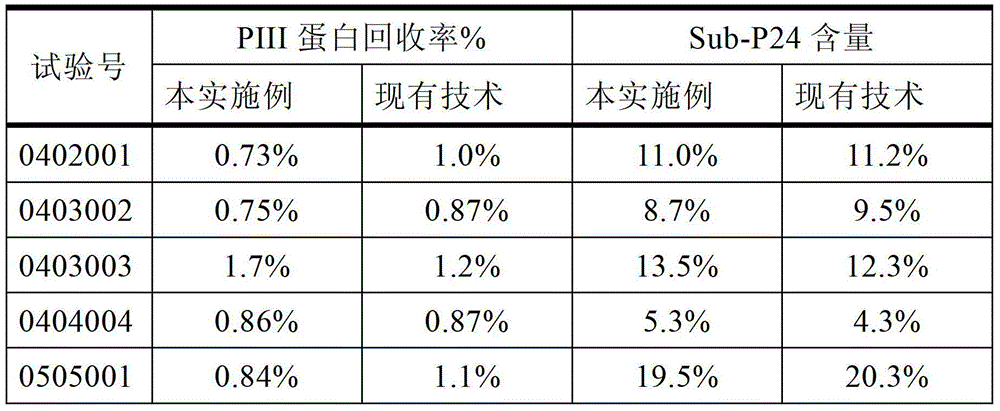
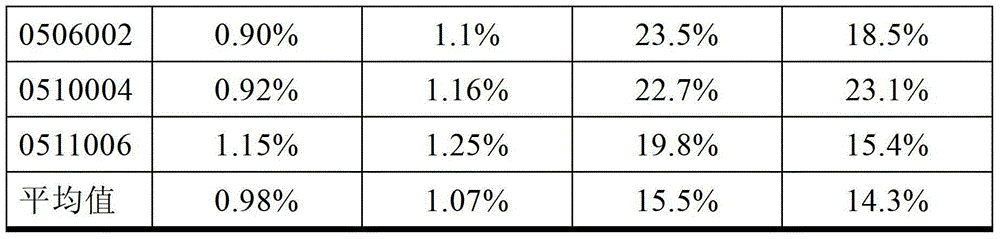
ActiveCN103333938AComply with GMP requirementsHigh degree of automationDigestive systemVirus peptidesAntigenUltrafiltration
The invention discloses a production method of a recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen. The method comprises the steps of: circularly removing triton from an extracted antigen by using XAD-4 column, so as to obtain triton-removed antigen sample liquid; loading macroporous silica gel with the pore size of 1,000 A and the particle size of 35-70 microns into a chromatographic column, and balancing by using phosphoric acid buffer solution with the pH of 7.6+ / -0.2; adjusting the pH of the triton-removed antigen sample liquid to be 7.6+ / -0.2 by using NaOH, then, loading a sample to the macroporous silica gel column; and cleaning impurities by using phosphoric acid buffer solution with the pH of 7.2+ / -0.2, carrying out eluting treatment on the impurity-cleaned macroporous silica gel column by using boric acid buffer solution with the pH of 8.7+ / -0.2 at the temperature of 47-49 DEG C, collecting eluate, and carrying out ultrafiltration and concentration on the eluate, thereby obtaining a clarified antigen. The invention further discloses the corresponding recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen, a hepatitis B vaccine and a production method of the hepatitis B vaccine. The production methods have the advantages that the probability of product contamination is reduced greatly, the labor intensity for laborers is reduced, the equipment investment and repairing cost are reduced, the space occupied by equipment is reduced, and the production time is shortened.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com