Technique for manufacturing anhydrous medicinal mirabilite
A manufacturing process, the technology of Glauber's salt, which is applied in the processing field of pharmaceutical chemicals, can solve the problems of unfavorable large-scale production, low purity of Glauber's salt, and large harvesting site, so as to reduce operating costs, ensure product purity, and increase production capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
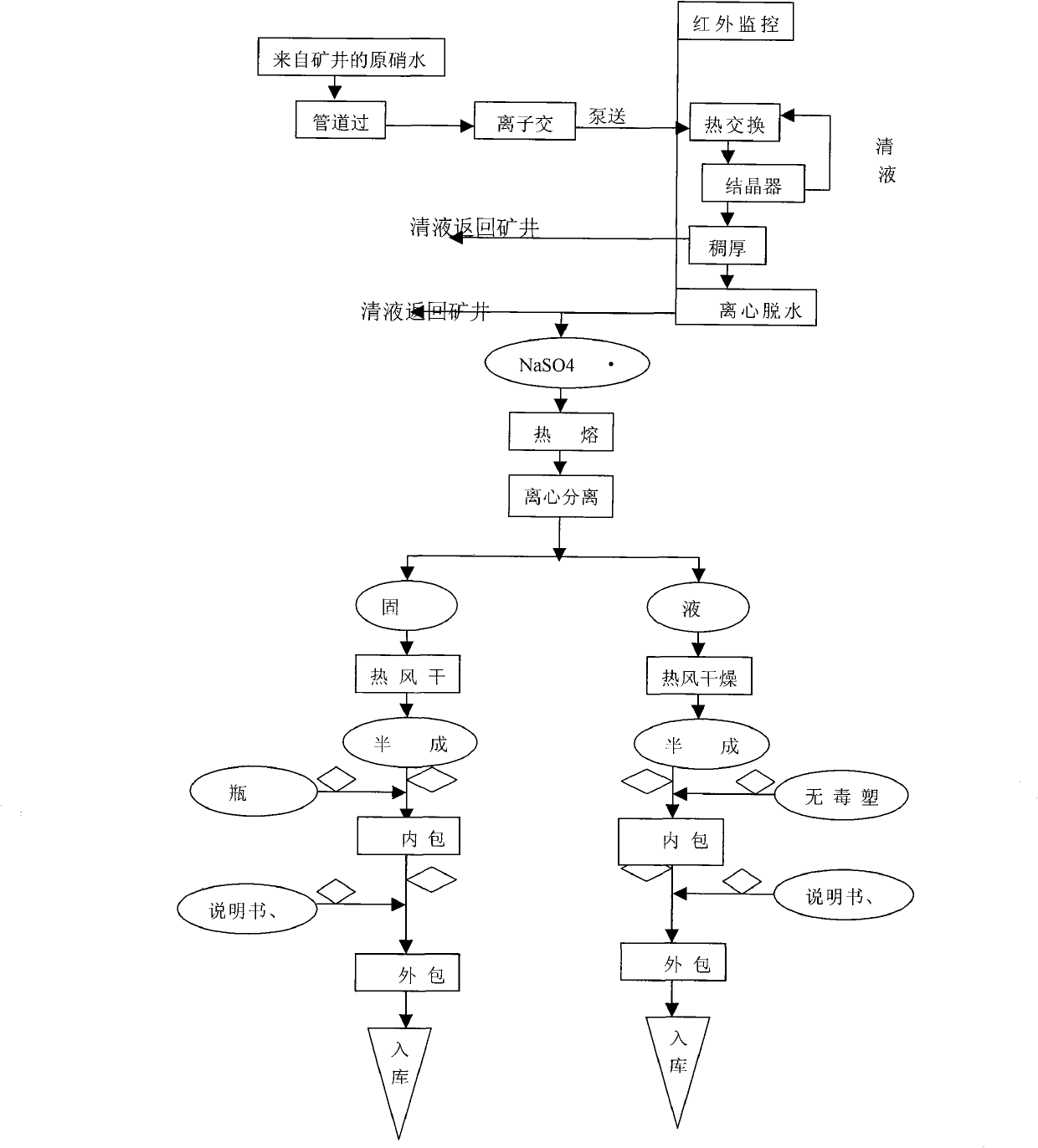
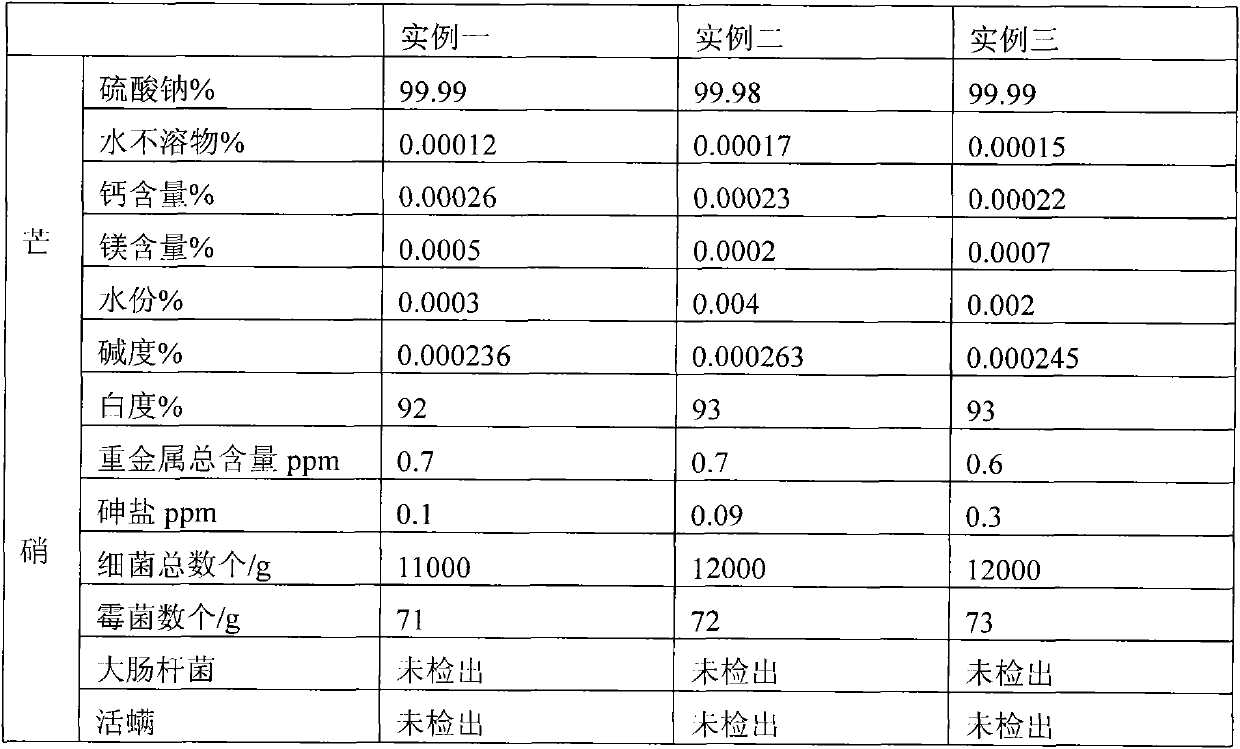
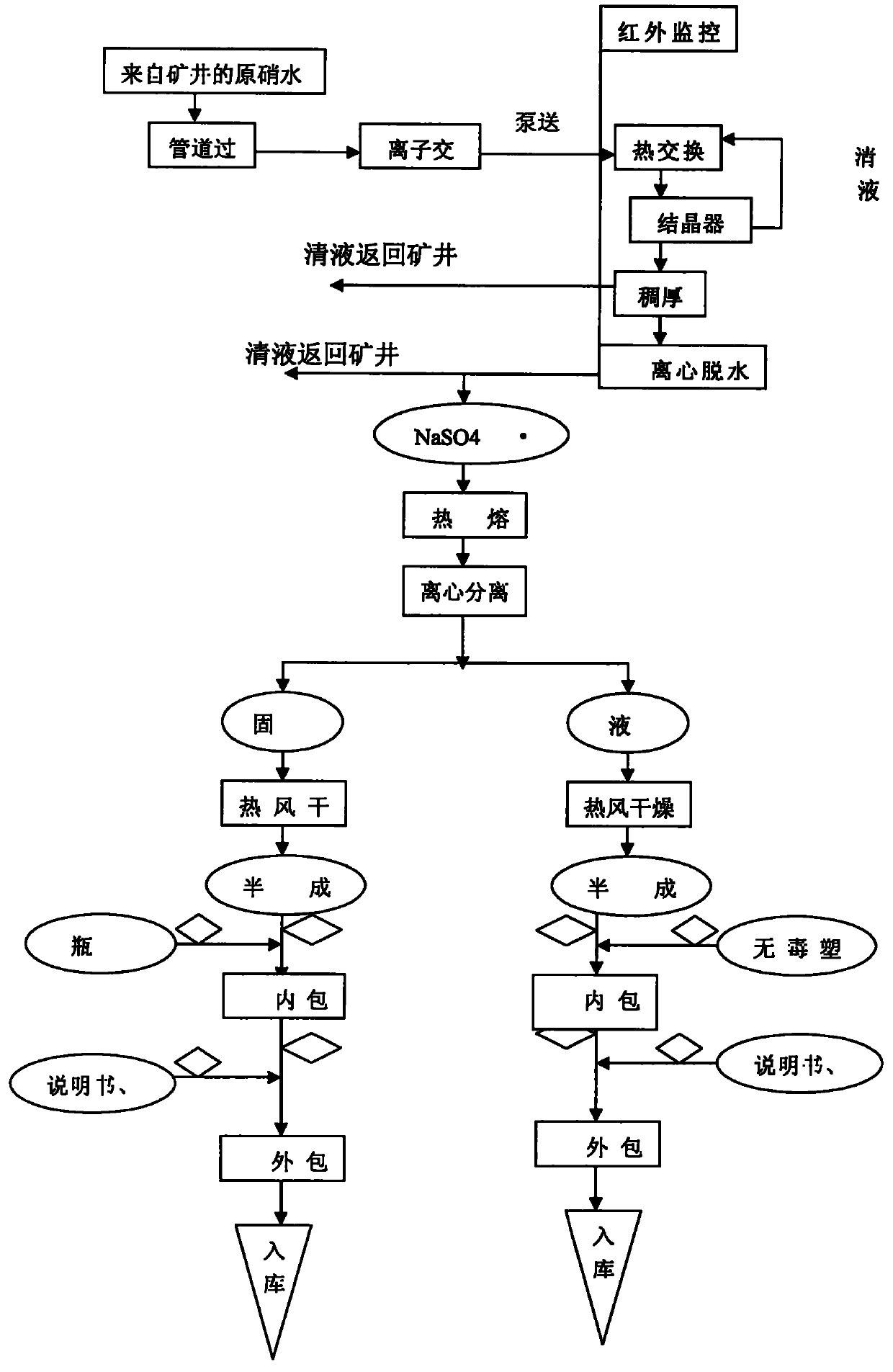
[0049] Through the monitoring of the online infrared computer automatic monitoring system and the regulation of the porous plate liquid balance flowmeter, it is ensured that the manufacturing process, production facilities and products of anhydrous medicinal thenardite (xuanming powder) meet the requirements of GMP, and the indoor air cleanliness in the production workshop is guaranteed. Reach the following indicators (planktonic bacteria 3 , sedimentation bacteria 3 , the number of suspended particles larger than 5μm 3 ) conditions, the 15-20% concentration of raw nitric water (Na 2 SO 4 Aqueous solution) through three-stage pipeline filter to remove mechanical impurities, and eight-stage ion exchanger to obtain refined nitric acid water with extremely low impurity content, which is input into continuous crystallization process system. In continuous crystallization system, there are heat exchangers and crystallizers And thickener and other equipment, the refined and purified ...
Embodiment 2
[0052] Through the monitoring of the online infrared computer automatic monitoring system and the regulation of the porous plate liquid balance flowmeter, it is ensured that the manufacturing process, production facilities and products of anhydrous medicinal thenardite (xuanming powder) meet the requirements of GMP, and the indoor air cleanliness in the production workshop reaches The following indicators (planktonic bacteria 3 , sedimentation bacteria 3 , the number of suspended particles larger than 5μm 3 ) conditions, the concentration of raw nitric water (Na 2 SO 4Aqueous solution) through three-stage pipeline filter to remove mechanical impurities, and eight-stage ion exchanger to obtain refined nitric acid water with extremely low impurity content, which is input into continuous crystallization process system. In continuous crystallization system, there are heat exchangers and crystallizers And thickener and other equipment, the refined and purified refined nitric water ...
Embodiment 3
[0055] Through the monitoring of the online infrared computer automatic monitoring system and the regulation of the porous plate liquid balance flowmeter, it is ensured that the manufacturing process and production facilities and products of anhydrous medicinal thenardite (Xuanming powder) meet the requirements of GMP, and the indoor air cleanliness in the production workshop reaches The following indicators (planktonic bacteria 3 , sedimentation bacteria 3 , the number of suspended particles larger than 5μm 3 ) conditions, the 15-20% concentration of raw nitric water (Na 2 SO 4 Aqueous solution) through three-stage pipeline filter to remove mechanical impurities, and eight-stage ion exchanger to obtain refined nitric acid water with extremely low impurity content, which is input into continuous crystallization process system. In continuous crystallization system, there are heat exchangers and crystallizers And thickener and other equipment, the refined and purified refined ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com