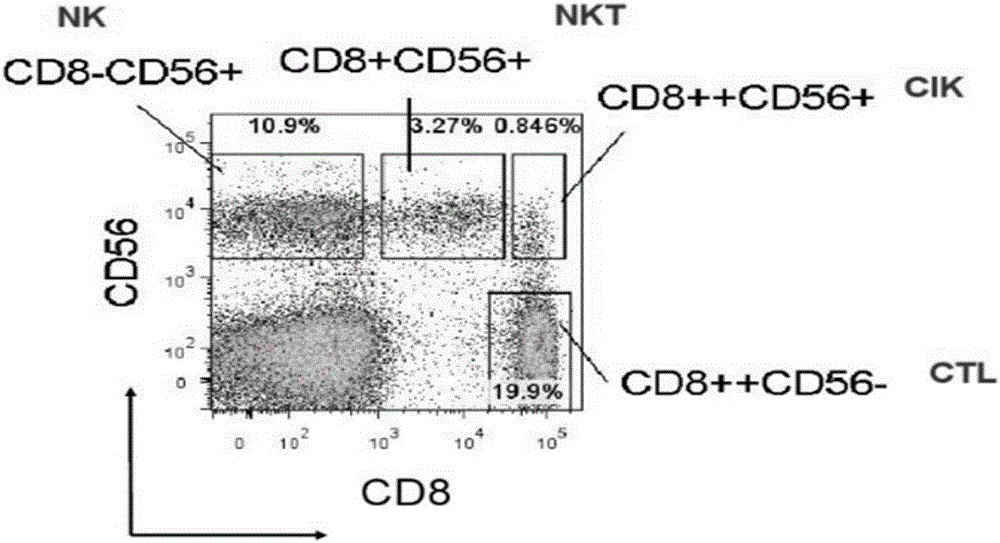
Method for preparing efficient clinical-level CD 56<+> group immune cell
A technology of immune cells and cells, applied in the biological field, can solve the problems of limited expansion and difficult to achieve clinical reinfusion, and achieve the effects of promoting differentiation and maturation, simple and easy technical operation, and reduced culture cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Isolation of mononuclear cells from peripheral blood or cord blood
[0038] 1. Aseptically collected 50ml of anticoagulated peripheral blood from the patient (male, 17 years old, acute myeloid leukemia, collected from the Department of Hematology, Siping Hospital, China Medical University with the consent of the patient);
[0039] 2. Transfer the collected blood sample to a 50ml centrifuge tube (Corning, product number: 430828), and use a Thermo 4KR centrifuge at room temperature to adjust the speed to 800g, and centrifuge for 10 minutes;
[0040] 3. Absorb the upper layer of plasma for later use during cultivation; dilute the blood at a ratio of 0.9% normal saline: remaining blood = 1:1, take two 50ml centrifuge tubes and add 15ml of lymphocyte separation solution (Tianjin Haoyang Biological Products Technology Co., Ltd. Company, article number: LTS1077006), slowly press the diluted blood: lymphocyte separation solution = 2:1 on the lymphocyte separation sol...
Embodiment 2
[0044] Embodiment 2: Anti-CD16 antibody coating culture vessel
[0045] Cell culture flasks (Corning, 75cm 2 , Cat. No.: 3275) was added to the coating solution containing anti-CD16 antibody (Biolegend, Cat. No.: 302013) (obtained by adding 5 μg antibody to 5 ml PBS buffer solution), incubated at room temperature for 4 h, and the final concentration of antibody was 1 μg / ml.
Embodiment 3
[0046] Example 3: Inoculation of mononuclear cells
[0047] Slowly rinse the culture flask coated with the antibody in Example 2 twice with 0.9% normal saline, resuspend the cells obtained in Example 1 with 45 ml of AIM-V serum-free medium (GIBCO, product number: 0870112-DK), and adjust the cell Density to 1×10 6 cells / ml and inoculated into culture flasks.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com