Fusion protein for preparing hepatitis B vaccine and its carrier
A fusion protein and hepatitis B vaccine technology, applied in the field of bioengineering, can solve the problem of inability to clear viruses or bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Design, Construction and Identification of Antibody Hepatitis B Vaccine
[0062] 1. Amplification of PreS2-S and murine IgG1 Fc-encoding genes
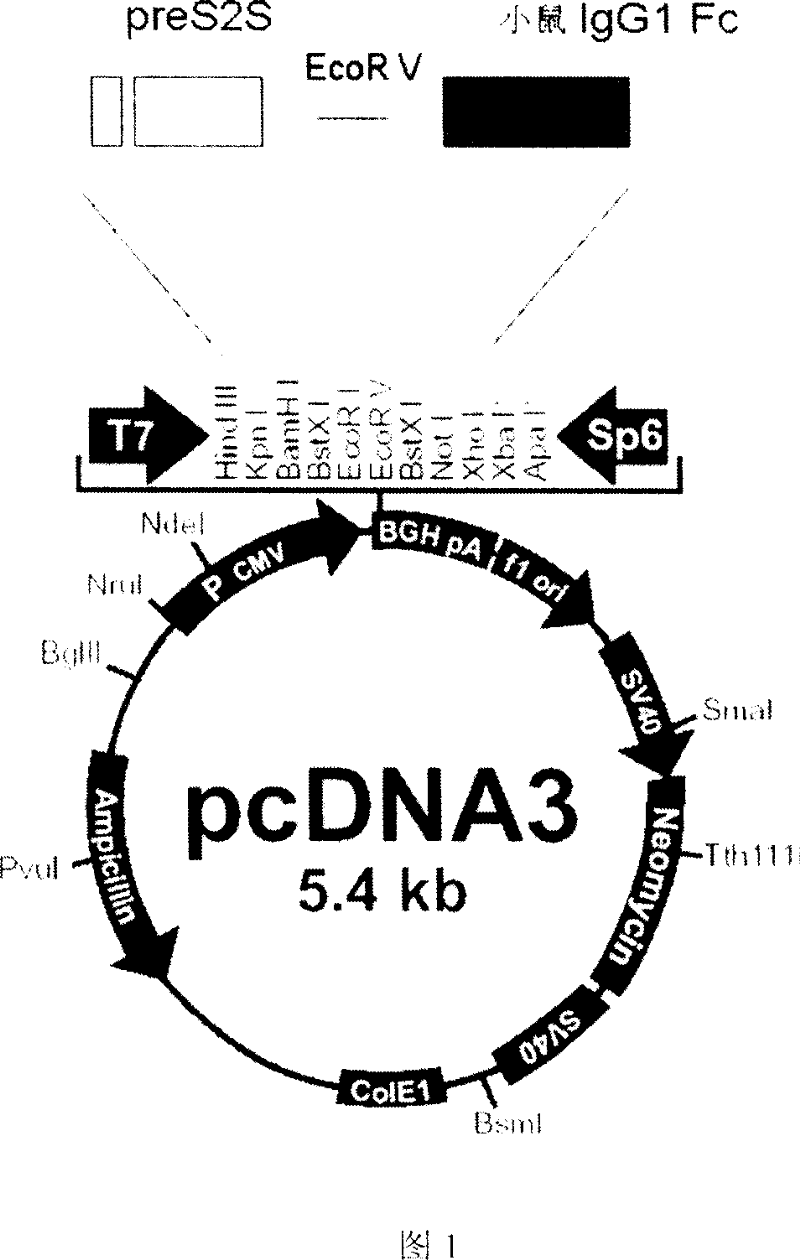
[0063] The antibodyized hepatitis B vaccine designed in the present invention is composed of hepatitis B surface antigen and mouse IgG1 Fc segment, and no linker is added in the middle. The N-terminus of the fusion molecule and the Fc segment are at the C-terminus of the fusion molecule, similar to a complete antibody molecule.
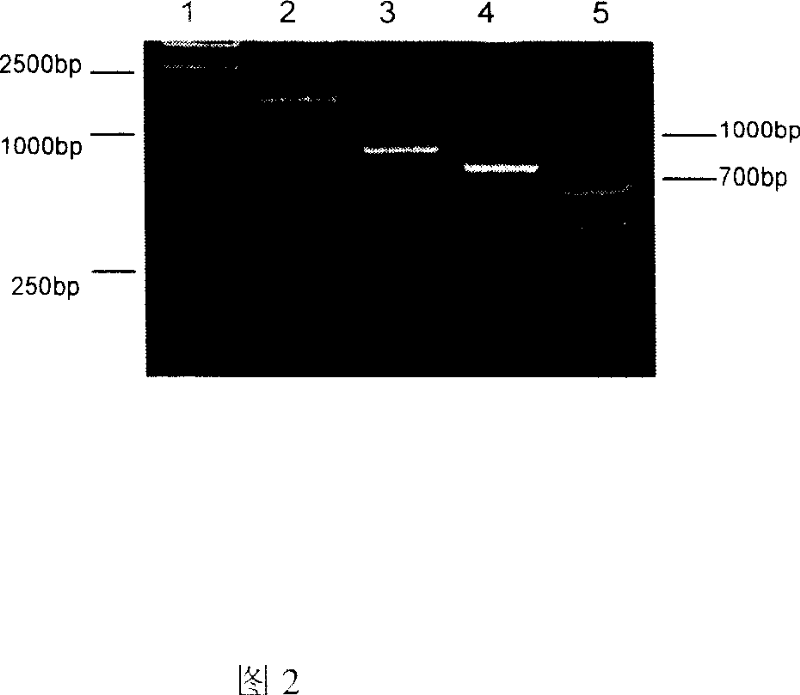
[0064] Hepatitis B surface antigen PreS2-S encoding gene was amplified by PCR with plasmid PEHH as template, and the primers adopted were shown in Table 1 (SEQ ID NO: 5 and SEQ ID NO: 6), and the enzyme cleavage site Hind was introduced while designing primers III and EcoR V.
[0065] The gene encoding the Fc segment of mouse IgG1 was amplified from mouse-derived hybridoma cells 520C9, and the cells were cultured in RPMI1640 (containing 10% calf serum, 1000u / ml penicillin, 100mg / ml strepto...
Embodiment 2
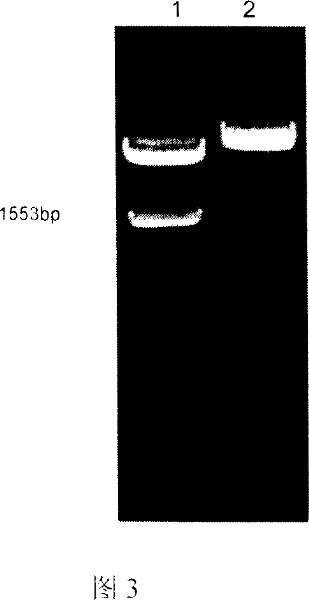
[0074] Example 2: In vitro transfection of PCMFS
[0075] According to Lipofectamine 2000 Instructions for eukaryotic transfection reagents: C2C12 cells were cultured in complete 1640 medium, and the cells were cultured at 5 × 10 a day before transfection. 5 C2C12 cells were added to a 35mm six-well plate and transfected when the cell density was 80%. Take 10 μl Lipofectamine 2000 Add to 90 μl of incomplete 1640 medium, which is solution A; take 2 μl (about 3-5 μg) of the plasmid purified by QIAGEN kit, which is solution B; add solution A to solution B, mix gently, and leave at room temperature. Act for 20-25 minutes to promote complex formation. During incubation at room temperature, the culture supernatant was aspirated, and the cells were washed once with PBS. The A and B complexes were added dropwise to the six-well plate, and then 800 μl of incomplete 1640 medium was added and mixed gently. 37℃ 5%CO 2 After 5 hours of culture, complete 1640 medium containing 20% N...
Embodiment 3
[0079] Example 3: Antibody Hepatitis B Vaccine Injected Through Muscle Gene
[0080] In this example, the plasmid PCMFS was injected.
[0081] The mice were intraperitoneally anesthetized with 0.75% pentobarbital sodium (about 120-150ul / mouse), took the abdominal position, and adjusted the posture of the legs to expose the tibialis anterior muscle. After disinfection, use a 1ml syringe with a plastic cannula to insert the needle vertically in the middle of the muscle. The insertion depth of the needle tip is determined by the cannula, which is about 2-3mm. Slowly advance, and the injection volume is 100ul (containing 100ug PCMFS plasmid). After the injection muscle expands, turn the needle 90°, stay for 5 seconds, and pull it out slowly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com