Culture medium additive for recombinant protein expression of humanized HEK293 cell line, recombinant hepatitis B vaccine and expression method
A medium additive, recombinant protein technology, applied in tissue culture, artificial cell constructs, chemical instruments and methods, etc., can solve the problem of low protein yield and achieve the effect of improving the expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Optimal design of HBsAg gene
[0027] Codon optimization was performed on the amino acid sequence disclosed in Genbank no: AAS20191.1 to obtain the optimized hepatitis B surface antigen gene (HBsAg gene), whose sequence is shown in SEQ ID NO.1.
Embodiment 2
[0028] Example 2 Construction of EGFP recombinant expression vector pIRES-EGFP
[0029] The present embodiment provides the construction method of the engineered bacterium that contains recombinant expression vector, comprises the following steps:
[0030] 1) PCR amplification of EGFP gene
[0031] Referring to the Enhanced green fluorescent protein (EGFP) gene sequence of the pEGFP-C1 vector (GenBank: U55763.1, bases 613-1332), design primers P1 and P2 (for amplifying 720bp EGFP gene DNA), primer 5 EcoRV and NheI restriction sites were introduced at the ' ends respectively, and the primer sequences were as follows (the restriction sites are underlined):
[0032] P1: 5′-CCG GATATC ATGGTGAGCAAGGGCGAGGAG-3'; as shown in SEQ ID NO.2;
[0033] P2: 5′-CTA ACCGGT GGACTTGTACAGCTCGTCCATGC-3'; shown in SEQ ID NO.3.
[0034] Using the pEGFP-C1 plasmid (purchased from Clontech, USA) as a template, primers P1 and P2 were used to amplify the EGFP gene. The reaction system is shown i...
Embodiment 3
[0044] Embodiment 3 Contains the construction of different promoter expression vectors
[0045] This embodiment provides a method for constructing recombinant expression vectors comprising different promoters, including the following steps:
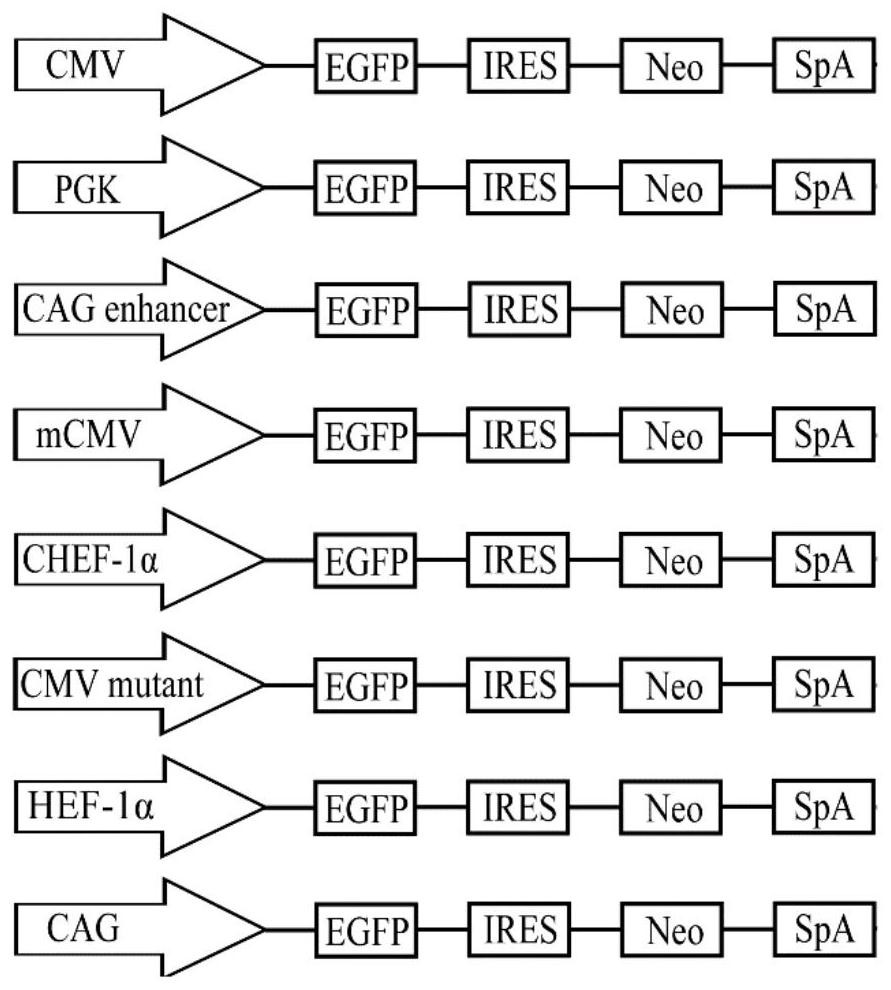
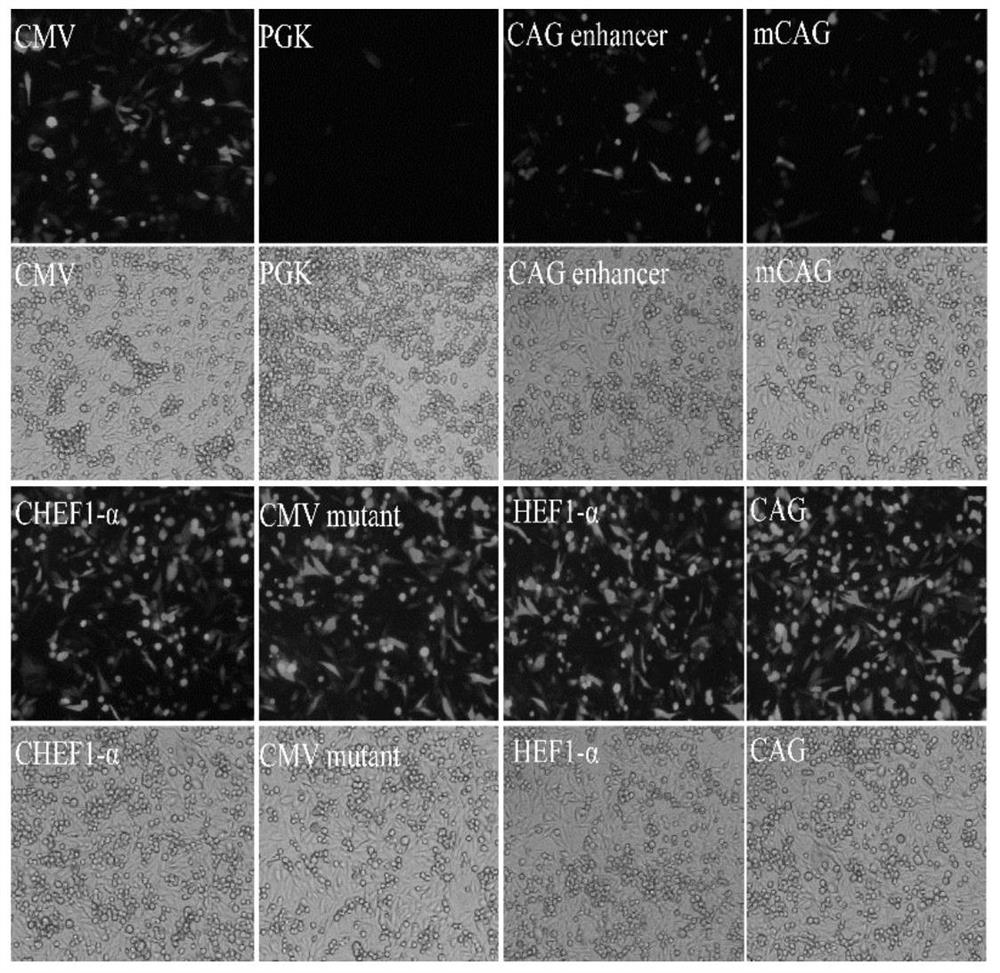
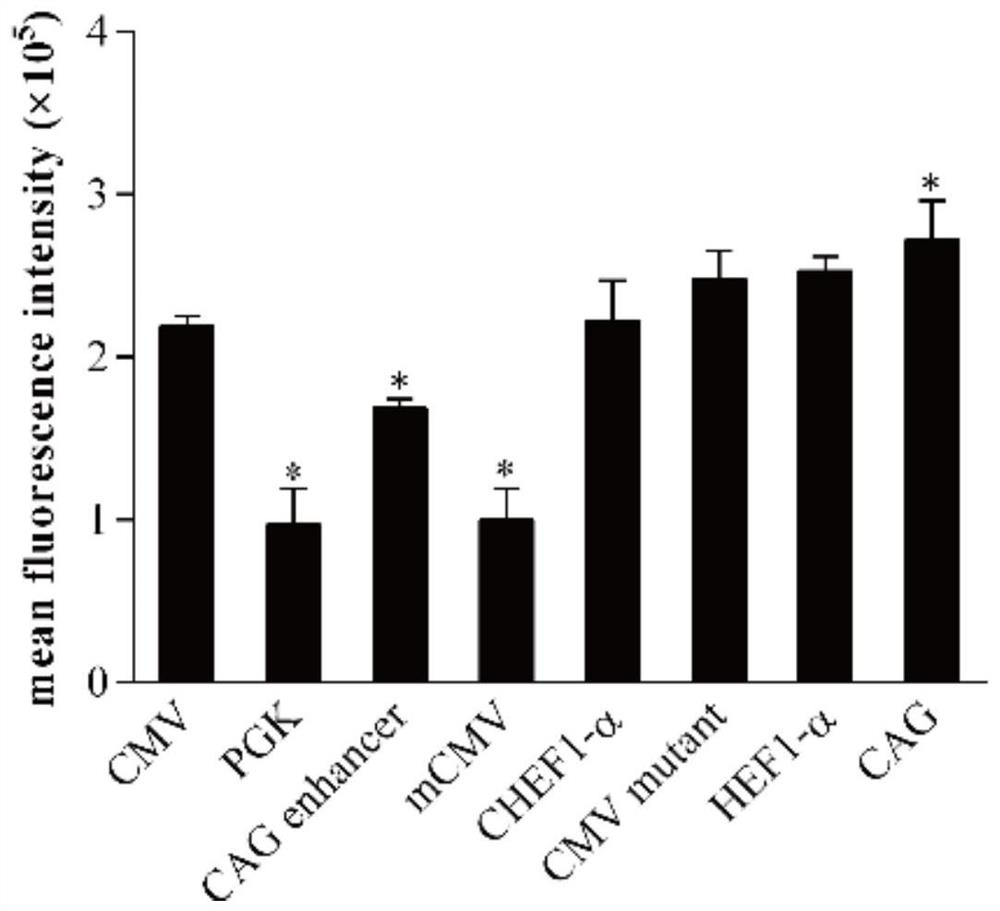
[0046] Synthetic PGK (GenBank: KJ175229.1), CAG enhancer (GenBank: AJ575208.1), mCMV (GenBank: KT343252.1), CHEF-1α (GenBank: KY447299.1), CMV mutant, HEF-1α (GenBank: AY188393.1) and CAG (GenBank: GU299216.1) seven promoter fragments. Use seamless cloning technology to replace the original promoter on pIRES-EGFP, such as figure 1 shown. The correctly constructed plasmids were named pIRES-PGK, pIRES-CAGen, pIRES-mCMV, pIRES-CHEF, pIRES-CMVmut, pIRES-HEF, pIRES-CAG, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com