Method used for preparing 2,4-dichloro-5-fluorobenzoyl chloride
A technology of fluorobenzoyl chloride and dichlorofluorobenzene, which is applied in the field of synthesizing 2,4-dichloro-5-fluorobenzoyl chloride with zeolite magnetic composite carrier catalyst, can solve the problem of excessively long process route and the yield of highly toxic chemicals. Difficult to achieve industrial production and other problems, to achieve the effect of facilitating sedimentation growth, improving catalytic activity, and good synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
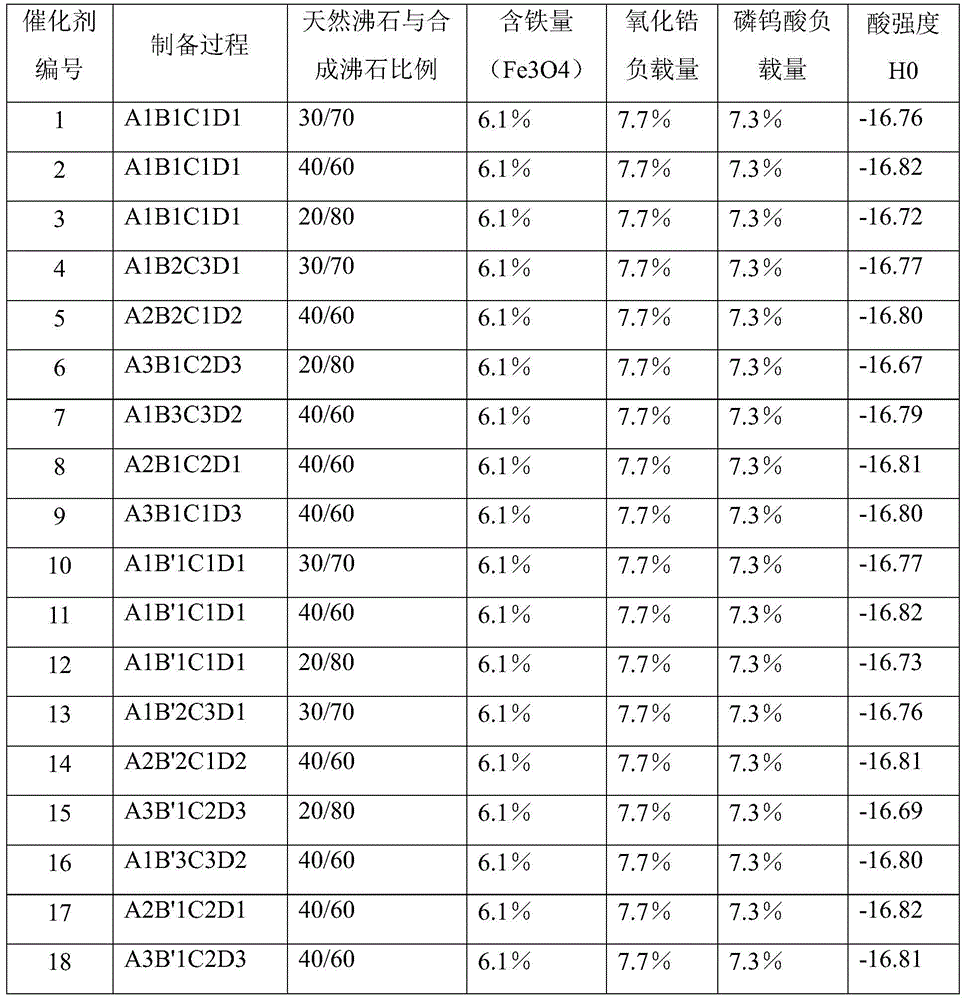
[0052] Preparation of composite zeolite solid super acid catalyst
[0053] Step (1): Zeolite pretreatment
[0054] A1
[0055] After roasting the mordenite carrier at 450°C, add an ammonium nitrate solution with an ammonium ion concentration of 1.0 mol / L for exchange treatment. The ratio of ammonium salt solution to zeolite liquid solids is 4:1, and the exchange time is 6 hours. Repeat 4 times, wash the exchanged mordenite with deionized water, then dry and calcinate again at 450°C;
[0056] Put the above-mentioned zeolite calcined again into H at a concentration of 6mol / L 2 SO 4 Soaked in the solution for 6 hours, washed with deionized water to neutrality, then dried and calcined at 450°C for 8 hours to obtain the pretreated carrier.
[0057] A2
[0058] After roasting the mordenite carrier at 650°C, add an ammonium sulfate solution with an ammonium ion concentration of 0.05 mol / L for exchange treatment. The ratio of ammonium salt solution to zeolite liquid solids is 3:1, and the excha...
Embodiment 1
[0110] Example 1: Preparation of 2,4-Dichloro-5-fluorobenzotrichloride
[0111] Add 194ml (2mol) of carbon tetrachloride and 33g (0.2mol) of 2,4-dichlorofluorobenzene to a 2L reactor, and then add 50g of the catalyst 8 in Table 1 to the reactor. The reaction is under reflux After reacting for 30 minutes, the reaction mixture was cooled to room temperature and poured into ice water. The organic layer was separated and subjected to chromatographic analysis. It was found that the product contained 94% of 2,4-dichloro-5-fluorobenzotrichloride.
Embodiment 2
[0112] Example 2: Preparation of 2,4-Dichloro-5-fluorobenzoyl chloride
[0113] Add 194ml (2mol) of carbon tetrachloride and 33g (0.2mol) of 2,4-dichlorofluorobenzene to a 2L reactor, and then add 16g of the catalyst 8 in Table 1 to the reactor, and the reaction is under reflux After reacting for 30 minutes, the reactor was cooled to room temperature, 200 ml of deionized water was added to the reaction system, and the reaction was carried out at 40°C for 1 hour. After the reaction, the fraction at 143-144°C (35mmHg) was collected by distillation to obtain 39.1 g Slightly yellow liquid with a yield of 86.0%.
[0114] The mass spectrometry and elemental analysis of the product obtained are as follows:
[0115] Mass spectrum: m / z: 225.92 (100.0%), 227.91 (95.9%), 229.91 (30.6%), 226.92 (7.6%), 228.92 (7.3%), 231.91 (3.3%), 230.91 (2.3%).
[0116] Elemental analysis results: C, 36.97; H, 0.89; Cl, 46.76; F, 8.35; O, 7.03. The molecular weight of the product is: 227.44.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com