A kind of preparation method of 3,4,5-trifluorobromobenzene compound
A technology of trifluorobromobenzene and trifluoroaniline, which is applied in the field of preparation of pesticides and pharmaceutical intermediates, can solve problems such as difficult separation of bromine, instability of diazonium liquid, and low reduction yield, so as to overcome difficult separation and recovery, and improve catalytic performance. Activity, beneficial to the effect of sedimentation growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
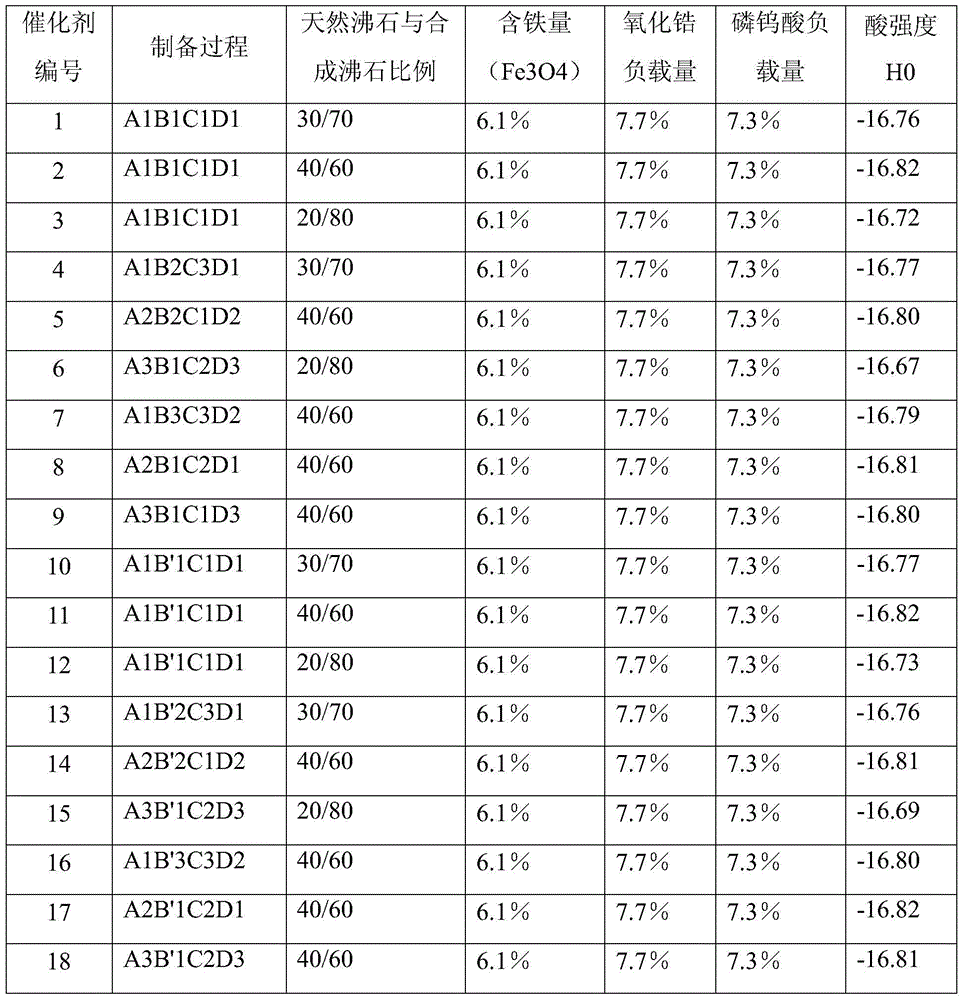
[0052] Preparation of Composite Zeolite Solid Superacid Catalyst
[0053] Step (1): Zeolite pretreatment
[0054] A1
[0055] After roasting the mordenite carrier at 450°C, add an ammonium nitrate solution with an ammonium ion concentration of 1.0mol / L for exchange treatment. The volume ratio of ammonium salt solution to zeolite liquid to solid is 4:1, and the exchange time is 6 hours. Repeat 4 times, wash the exchanged mordenite with deionized water, then dry it and bake it again at 450°C;
[0056] Put the aforementioned zeolite that has been roasted again into H2 with a concentration of 6mol / L 2 SO 4 Soak in the solution for 6 hours, wash with deionized water until neutral, dry, and bake at 450° C. for 8 hours to obtain a pretreated carrier.
[0057] A2
[0058] After roasting the mordenite carrier at 650°C, add an ammonium sulfate solution with an ammonium ion concentration of 0.05mol / L for exchange treatment. The volume ratio of ammonium salt solution to zeolite liqui...
Embodiment 1
[0110] Embodiment 1: the preparation of 3,4,5-trifluorobromobenzene
[0111] 500 milliliters of water, 147 grams of 2,3,4-trifluoroaniline, 214.5 grams of glacial acetic acid (98% in mass concentration, 3.5 moles) were put into the reactor, and sulfuric acid was added dropwise at 25-30°C to control the temperature in the reactor. 450 grams (mass concentration is 98%, 4.5 moles), control the temperature in the reactor to react at 25~30 ℃ for 1 hour after the addition is completed, add ammonium carbonate to adjust the pH of the reaction system to 9-10. Then add 80 grams of the catalyst 2 in the aforementioned Table 1 and 0.4 moles of bromine, control the temperature in the reactor to be 35-40° C., add hydrogen peroxide and water for dilution dropwise, and keep the reaction under stirring after the dropwise addition is completed. During the heat preservation reaction, the product mixture obtained from the heat preservation reaction is sampled, the sample is neutralized to PH=6~8 ...
Embodiment 2
[0116] Embodiment 2: the preparation of 3,4,5-trifluorobromobenzene
[0117] Example 1 was repeated except that 150 grams of catalyst 2 in Table 1 was added to obtain 181.1 grams of 3,4,5-trifluorobromobenzene with a yield of 88.7%. The by-product 1,2-dibromo-3,4,5-trifluorobenzene was not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com