A kind of preparation method of 2,4-dichloro-5-fluorobenzoyl chloride
A technology for fluorobenzoyl chloride and dichlorofluorobenzene is applied in the field of synthesizing 2,4-dichloro-5-fluorobenzoyl chloride with a zeolite magnetic composite carrier catalyst, which can solve the problem that it is difficult to realize industrialized production, and the process route is too long and highly toxic Chemical yield and other issues, to achieve the effect of improving catalytic activity, conducive to sedimentation growth, and good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
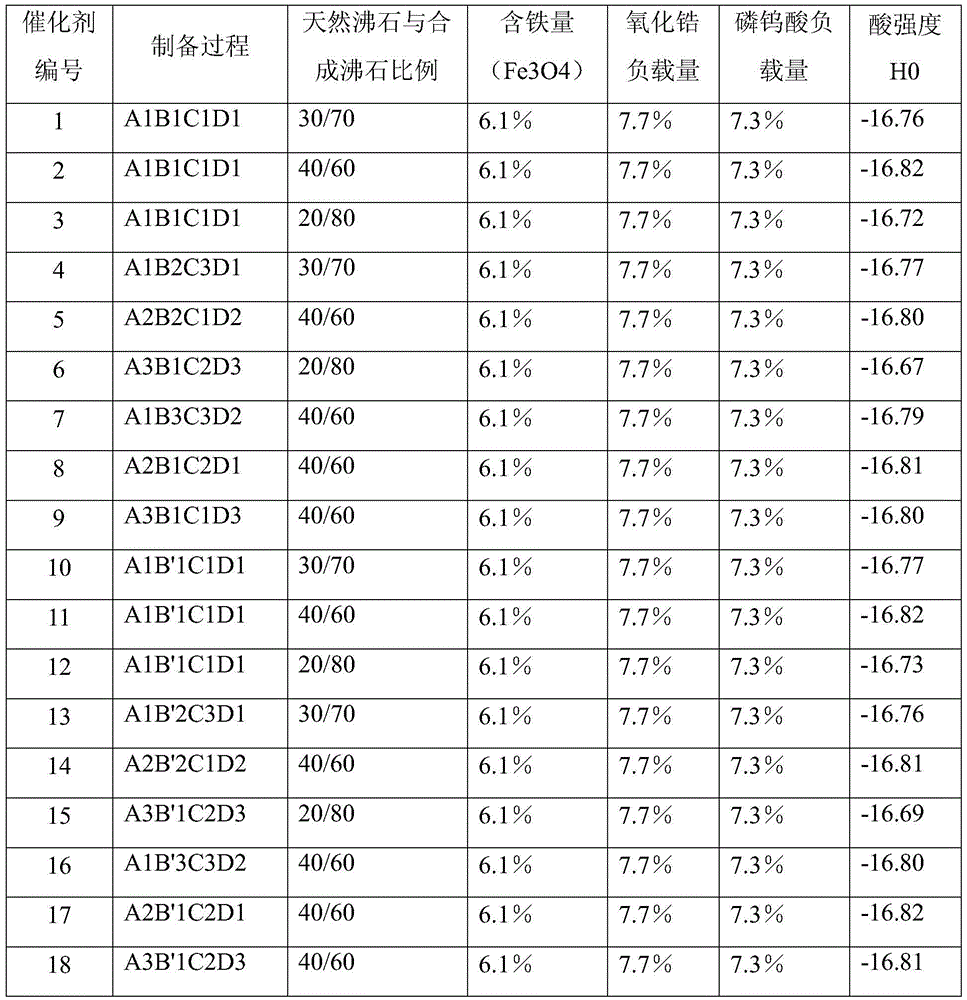
[0051] Preparation of Composite Zeolite Solid Superacid Catalyst
[0052] Step (1): Zeolite pretreatment
[0053] A1
[0054] After roasting the mordenite carrier at 450°C, add an ammonium nitrate solution with an ammonium ion concentration of 1.0mol / L for exchange treatment. The volume ratio of ammonium salt solution to zeolite liquid to solid is 4:1, and the exchange time is 6 hours. Repeat 4 times, wash the exchanged mordenite with deionized water, then dry it and bake it again at 450°C;
[0055] Put the aforementioned zeolite that has been roasted again into H2 with a concentration of 6mol / L 2 SO 4 Soak in the solution for 6 hours, wash with deionized water until neutral, dry, and bake at 450° C. for 8 hours to obtain a pretreated carrier.
[0056] A2
[0057] After roasting the mordenite carrier at 650°C, add an ammonium sulfate solution with an ammonium ion concentration of 0.05mol / L for exchange treatment. The volume ratio of ammonium salt solution to zeolite liqui...
Embodiment 1
[0109] Embodiment 1: Preparation of 2,4-dichloro-5-fluorobenzotrichloride
[0110] In the reactor of 2L, add 194ml (2mol) carbon tetrachloride and 33 grams (0.2mol) 2,4-dichlorofluorobenzene, then add the catalyst 8 in 50 grams of aforementioned Table 1 in reactor, react at reflux The reaction mixture was reacted at room temperature for 30 minutes, and then the reaction mixture was cooled to room temperature and poured into ice water. The organic layer was separated and subjected to chromatographic analysis, and it was found that the product contained 94% of 2,4-dichloro-5-fluorobenzotrichloride.
Embodiment 2
[0111] Embodiment 2: Preparation of 2,4-dichloro-5-fluorobenzoyl chloride
[0112] In the reactor of 2L, add 194ml (2mol) carbon tetrachloride and 33 grams (0.2mol) 2,4-dichlorofluorobenzene, then add catalyzer 8 in 16 grams of aforementioned Table 1 in reactor, react at reflux The reaction was carried out at room temperature for 30 minutes, then the reactor was cooled to room temperature, 200 milliliters of deionized water was added to the reaction system, and the reaction was carried out at 40° C. for 1 hour. After the reaction, the fraction at 143-144° C. (35 mmHg) was collected by distillation to obtain 39.1 grams Light yellow liquid, yield 86.0%.
[0113] The resulting product was subjected to mass spectrometry and elemental analysis, and the results were as follows:
[0114] Mass spectrum: m / z: 225.92 (100.0%), 227.91 (95.9%), 229.91 (30.6%), 226.92 (7.6%), 228.92 (7.3%), 231.91 (3.3%), 230.91 (2.3%).
[0115] Elemental analysis results: C, 36.97; H, 0.89; Cl, 46.76;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com