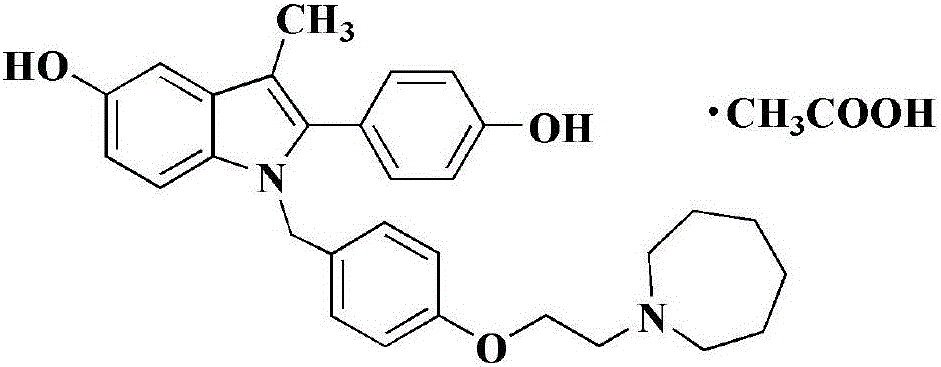
Pharmaceutical analysis method for efficiently measuring bazedoxifene acetate and impurities of bazedoxifene acetate
A bazedoxifene acetate, high performance liquid chromatography technology, applied in the directions of analysis materials, measuring devices, material separation, etc., can solve the problems of too long analysis time, ineffective separation, single gradient change procedure, etc., and achieves high sensitivity, The effect of quality control guarantee and good versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 1. Chromatographic conditions
[0060] Instrument: High Performance Liquid Chromatography—UV Detector
[0061] Chromatographic column: use octadecylsilane bonded silica gel as filler (Xbridge C18, 4.6×150mm, 3.5μm);
[0062] Mobile phase A: phosphate buffer with a pH value of 8.0-acetonitrile (61:39); wherein, the phosphate buffer with a pH value of 8.0 adjusts the pH by adding an appropriate amount of 10% phosphoric acid solution to a 0.01mol / L dipotassium hydrogen phosphate solution Values up to 8.0 are obtained.
[0063] Mobile phase B: 0.5% phosphoric acid aqueous solution-acetonitrile (10:90);
[0064] gradient elution procedure
[0065]
[0066] Flow rate: 1.0ml / min; Column temperature: 40°C; Detection wavelength: 220nm; Injection volume: 10μl
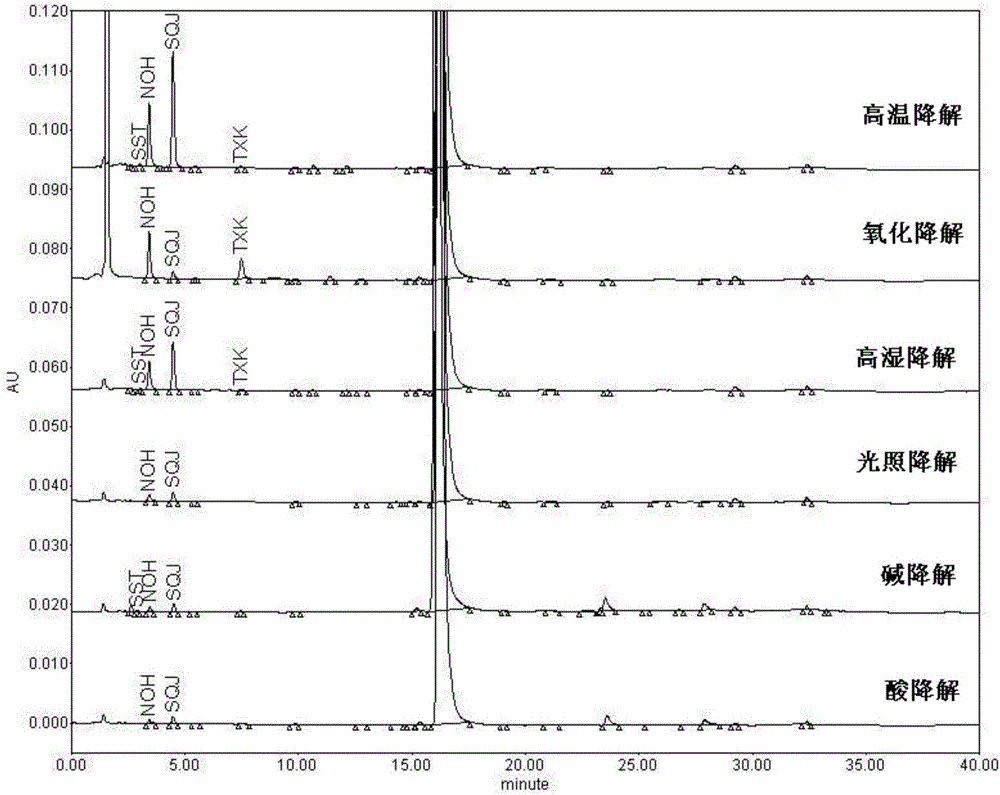
[0067] 2. Validation of analytical methods:
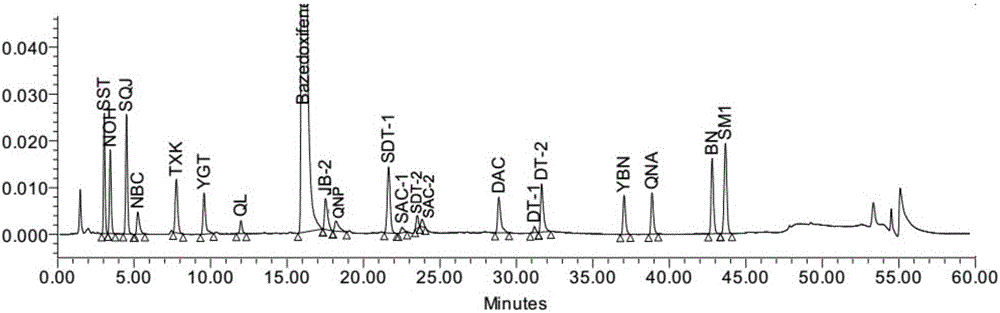
[0068] (1) System suitability / impurity location
[0069] System suitability solution: take bazedoxifene acetate, impurity SST, impurity NOH, impurity SQJ, impurity N...
Embodiment 2
[0083] The preparation of embodiment 2 impurity compound QNA
[0084]
[0085] 6.0 g of compound BN and 3.4 g of compound SM2 were dissolved in 100 mL of acetonitrile, and the mixture was stirred at room temperature for 24 h. After the reaction was completed, suction filtration was performed, and the obtained filtrate was evaporated to dryness under reduced pressure, and the residue was subjected to column chromatography to obtain 2.0 g of impurity QNA;
[0086] ESI(+): m / z 882.39;
[0087] [M+1] + ; 1 H NMR: (400MHz, DMSO-d6) δ = 11.5-11.6 (s, 1H), 7.623 (d, 2H, J = 8.4Hz), 7.491 (dd, 4H, J = 1.6Hz, J = 7.2Hz), 7.434-7.320(m,8H),7.235(d,1H,J=9.2Hz),7.156-7.099(m,5H),6.902(d,2H,J=8.4Hz),6.839-6.811(m,3H) ,5.206(s,2H),5.157(d,4H,J=12.0Hz),4.681(s,2H),4.524-4.499(m,4H),3.602-3.400(m,6H),3.250-3.200(m ,2H),3.1-3.2(s,4H),2.175(s,3H),1.943-1.883(m,2H),1.812(s,6H),1.682-1.638(m,2H),1.559(s,6H ).
Embodiment 3
[0088] The preparation of embodiment 3 impurity compound QNP
[0089]
[0090] Disperse 1.0 g of compound QNA in a mixed system of 20 mL of ethanol and 5 mL of ethyl acetate, add 0.1 g of 10% palladium carbon under nitrogen protection, replace the reaction system with hydrogen for 3 times, and stir the mixture at room temperature until TLC shows that the reaction is complete, and the reaction liquid suction filtration, the filter cake was washed with 30ml ethyl acetate, the organic phases were combined, and the solvent was evaporated to dryness to obtain a crude product, which was purified by chromatographic column chromatography to obtain 0.5g impurity compound QNP;
[0091] ESI(+): m / z 702.20;
[0092] [M] + ; 1 H NMR: (400MHz,DMSO-d6)δ=11.360(d,1H,J=6.4Hz),9.862(s,1H),8.798(s,1H),7.585(d,2H,J=8.8Hz), 7.186(d, 2H, J=8.4Hz), 7.093(dd, 3H, J=2.0Hz, J=8.8Hz), 6.914-6.820(m, 7H), 6.607(dd, 1H, J=2.4Hz, J =8.8Hz), 5.142(s, 2H), 4.635(s, 2H), 4.471(s, 2H), 3.580-3.436(m, 8H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com