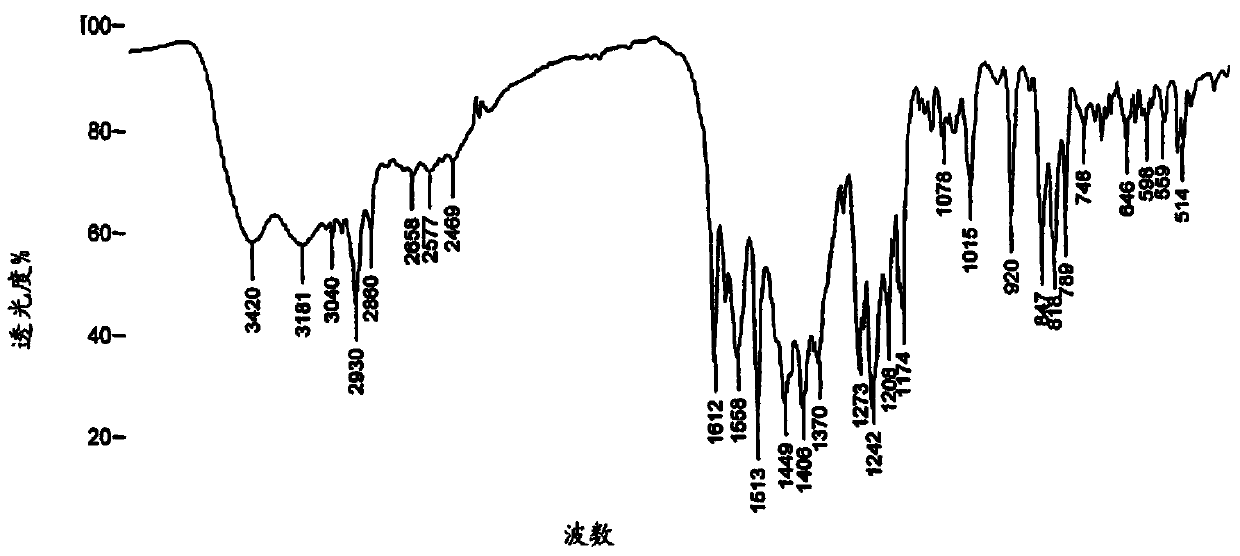
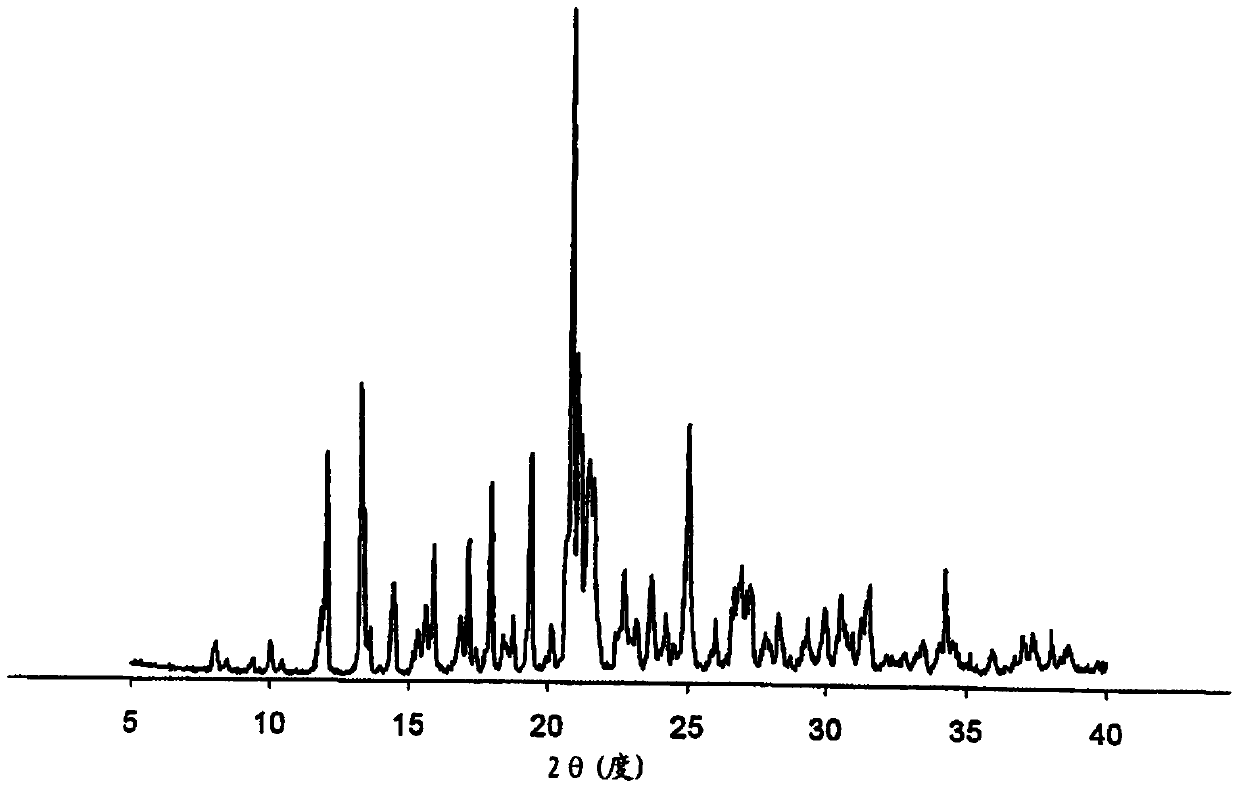
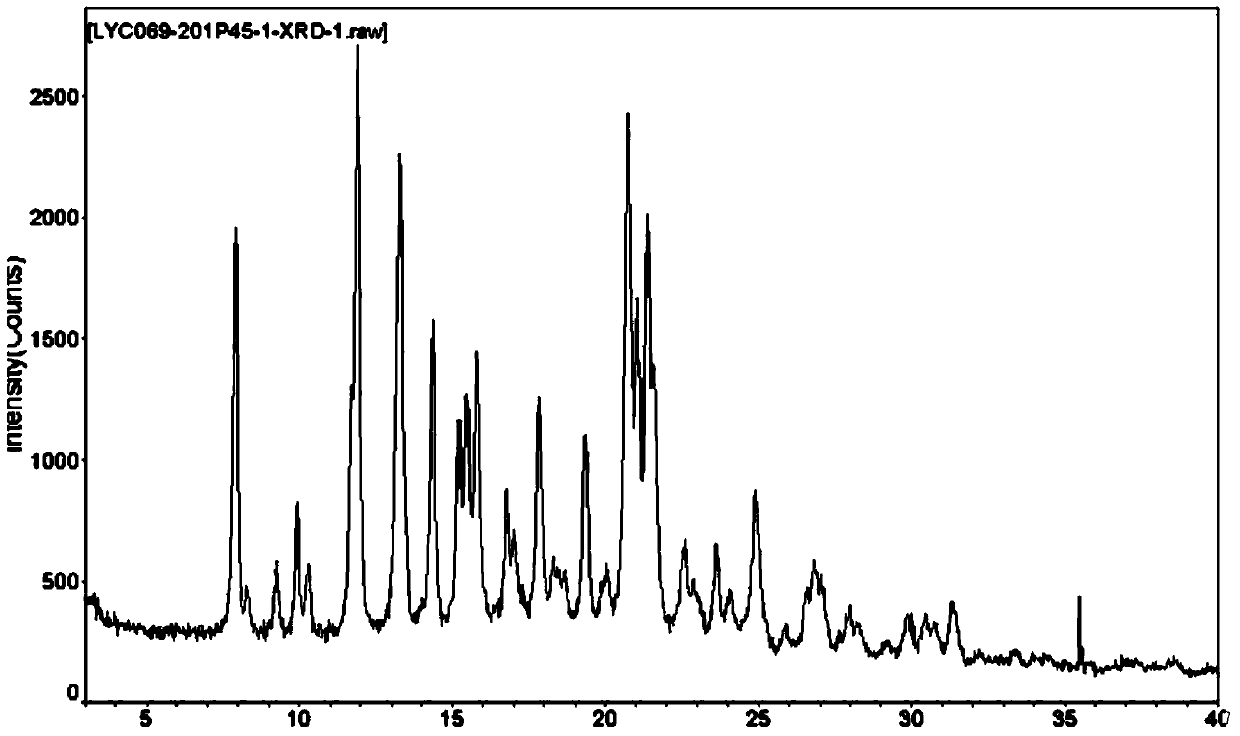
Preparation method of bazedoxifene acetate polycrystalline type B
A technology of bazedoxifene acetate and its polymorphic forms, which is applied in the field of pharmacy, can solve the problems of no public preparation of polymorphic form B, no detailed description of the salt-forming process, and unsuitability for large-scale production, so as to shorten the production cycle and cost Salt crystallization time is short, suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 4.0g of bazedoxifene free base and 12.0g of methyl tert-butyl ether into a 250mL three-neck flask, control the temperature and stir to dissolve at 18°C, add 1.1g (3eq) of glacial acetic acid and continue to insulate and stir for 1 hour, then cool down to Suction filtration at 10°C, and the filter cake was washed with an appropriate amount of methyl tert-butyl ether to obtain bazedoxifene acetate polymorph B solid. The solid was vacuum-dried at 35°C to obtain 3.19 g of bazedoxifene acetate, with a yield of 98.0%.
Embodiment 2
[0036] Weigh 4.0g of bazedoxifene free base and 40.0g of methyl tert-butyl ether in a 250mL three-neck flask, stir and dissolve under temperature control at 27°C, add 0.37g (1eq) of glacial acetic acid and continue to insulate and stir for 2 hours before cooling down to Suction filtration at 20°C, and the filter cake was washed with an appropriate amount of methyl tert-butyl ether to obtain bazedoxifene acetate polymorph B solid. The solid was vacuum-dried at 40°C to obtain 3.16 g of bazedoxifene acetate, with a yield of 97.0%.
Embodiment 3
[0038] Weigh 4.0g of bazedoxifene free base and 160.0g of methyl tert-butyl ether in a 250mL three-necked flask, control the temperature and stir to dissolve at 35°C, add 0.73g (2eq) of glacial acetic acid and continue to insulate and stir for 3 hours before cooling down to Suction filtration at 10°C, and the filter cake was washed with an appropriate amount of 4-methyl-2-pentanone to obtain bazedoxifene acetate polymorph B solid. The solid was vacuum-dried at 40°C to obtain 3.18 g of bazedoxifene acetate, with a yield of 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com