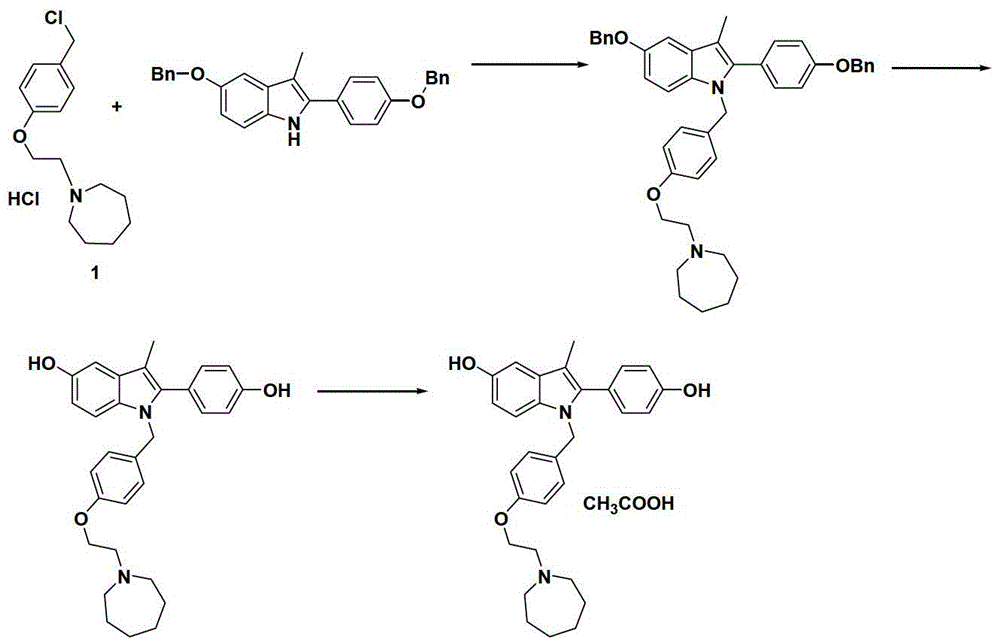
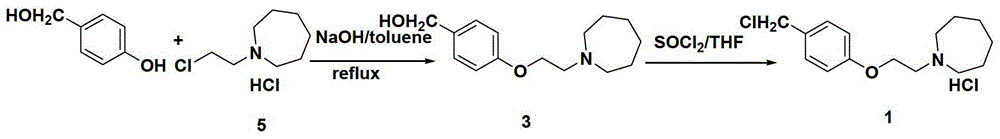
The preparation method of bazedoxifene acetate intermediate
A technology for bazedoxifene acetate and intermediates, which is applied in the field of preparation of bazedoxifene acetate intermediates, and can solve problems such as poor product purity, high toxicity, and poor atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
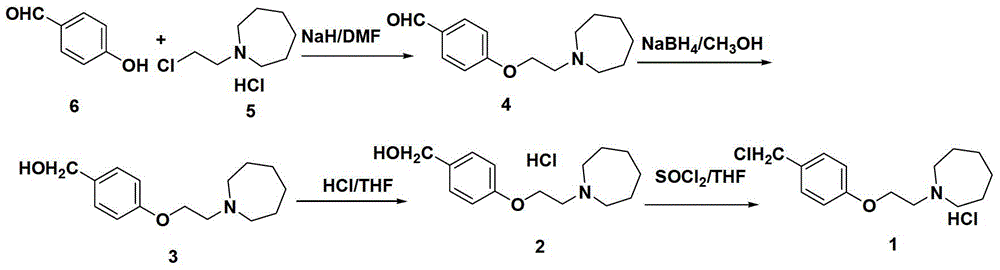
Embodiment 14-
[0056] Preparation of Example 14-[2-(cyclohexylimino-1-yl)ethoxy]benzyl alcohol 3
[0057] Under the protection of nitrogen, add sodium tert-butoxide (24.2g, 253mmol) into DMF (150mL), stir for 30min, add a solution of p-hydroxybenzaldehyde (16.6g, 136mmol) in DMF (80mL) dropwise at 0°C, dropwise, keep warm (0°C) Stir for 30min. 2-(Cyclohexylimino)ethyl chloride hydrochloride (25g, 124mmol) was added at 0°C. After the addition was complete, the temperature was raised to 25°C for 4h. TLC tracking showed that the reaction of 2-(cyclohexylimino)ethyl chloride hydrochloride was complete, methanol (110mL) was added to the reaction solution, the temperature dropped to 0°C, and sodium borohydride (2.7g, 69.6mmol) was added in batches , After addition, react at 25°C for 2 hours. The reaction solution was poured into ice water (200 mL), extracted with ethyl acetate (100 mL×3), washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under ...
Embodiment 24-
[0058] Preparation of Example 24-[2-(cyclohexylimino-1-yl)ethoxy]benzyl alcohol 3
[0059] Under the protection of nitrogen, sodium hydride (10.1g, 253mmol) was added to DMF (80mL), stirred for 10min, and a solution of p-hydroxybenzaldehyde (16.6g, 136mmol) in DMF (80mL) was added dropwise at 0°C. ℃) and stirred for 30 minutes. 2-(Cyclohexylimino)ethyl chloride hydrochloride (25g, 124mmol) was added at 0°C. After the addition was complete, the temperature was raised to 25°C for 2h. TLC tracking showed that the reaction of 2-(cyclohexylimino)ethyl chloride hydrochloride was complete, ethanol (100mL) was added to the reaction solution, the temperature dropped to 0°C, and sodium borohydride (2.7g, 69.6mmol) was added in batches After the addition was completed, the reaction was carried out at room temperature at 25°C for 2 hours. The reaction solution was poured into ice water (200 mL), extracted with ethyl acetate (100 mL×3), washed with saturated sodium chloride solution, dri...
Embodiment 34-
[0060] Preparation of Example 34-[2-(cyclohexylimino-1-)ethoxy]benzyl chloride hydrochloride 1
[0061] Under the protection of nitrogen, add anhydrous methanol (0.7mL, 17mmol) into the three-necked flask, lower the temperature to 0°C, slowly add acetyl chloride (1.2mL, 17mmol) dropwise, keep warm (0°C) and stir for 30min, and 4-[2 -(Cyclohexylimin-1-yl)ethoxy]benzyl alcohol (4.3g, 17mmol) in THF (10mL) was slowly added dropwise to the above solution, and a white solid was formed. When the solid no longer increases, add thionyl chloride (1.8mL, 25mmol) dropwise at the same temperature. After the drop is completed, the temperature is raised to 50°C, and the solid gradually dissolves. TLC tracking shows that 4-[2-(cyclohexylimine-1- Base) ethoxyl] benzyl alcohol hydrochloride is completely reacted, concentrated under reduced pressure to half of the solution volume, refrigerated overnight at 0°C, filtered with suction, and dried to obtain 4.5 g of light yellow solid 4-[2-(cyclohe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com