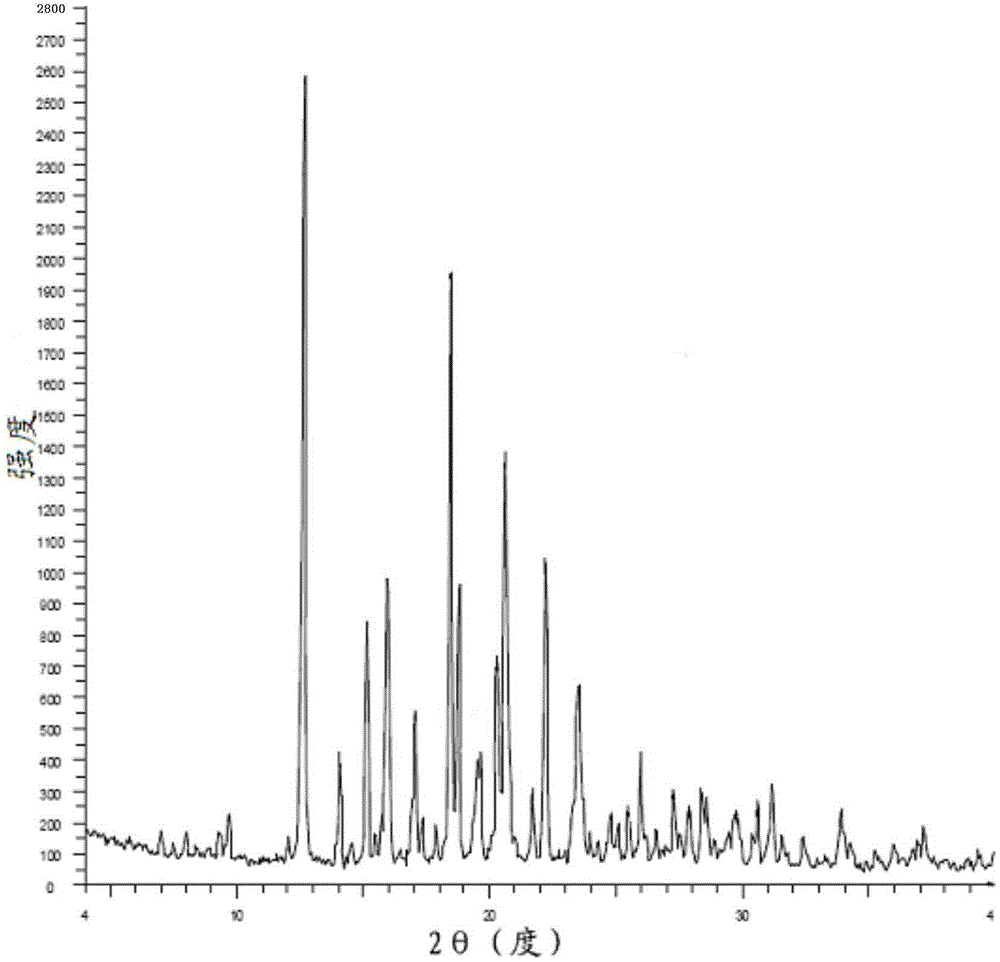
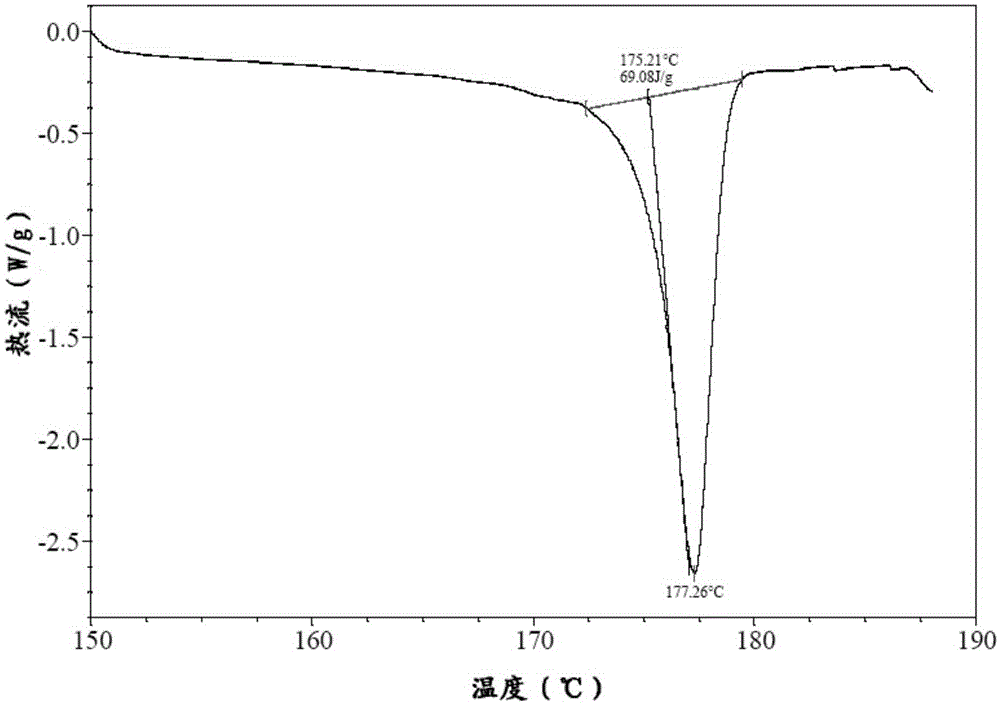
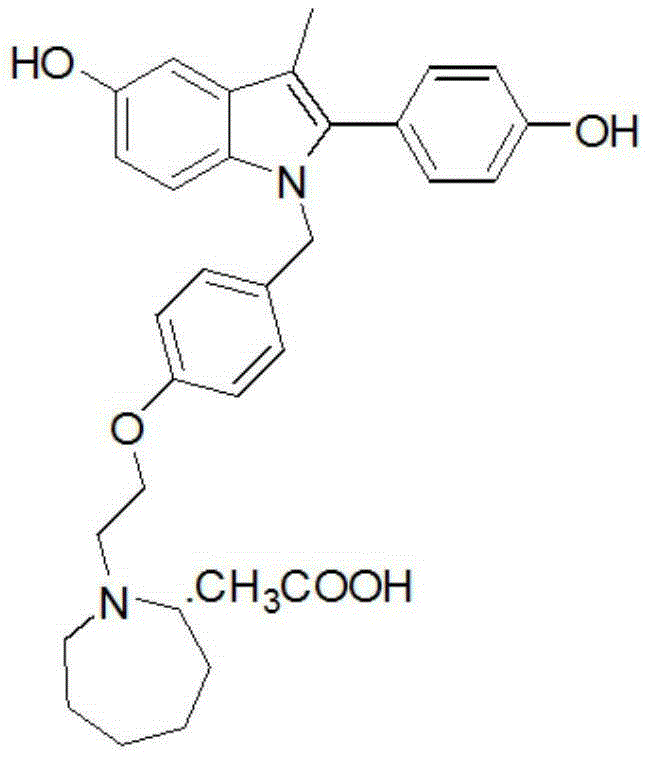
Preparation method of bazedoxifene acetate and crystal form A
A technology of bazedoxifene acetate and crystal form, applied in the field of medicinal chemistry, can solve the problems of poor reproducibility, difficult control of crystal form purity, unfavorable solvent recovery, etc., and achieves high crystal form purity, reduced risk, and easy recovery and reuse. The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 bazedoxifene acetate
[0027] The reaction formula is as follows:
[0028]
[0029] Add 540g of tetrahydrofuran, 180g of the compound of formula A, 360g of ethanol, 45g of ammonium formate and 9g of 5% palladium carbon catalyst into a 2000ml three-necked flask in turn under stirring; heat up to 65°C and react for 16-28 hours until TLC shows that the raw materials and monobenzyl The point disappears, (or the residual monobenzyl compound detected by HPLC≤0.1%). Filtrate under reduced pressure, rinse the filter cake with 900g of ethyl acetate, put the filtrate into a 5L separatory funnel, wash with 600g×3 water, add 25.0g of acetic acid to the organic layer while stirring in a 2L single-necked bottle, and solids precipitate out in about 10 minutes, then After stirring for 20 minutes, cool in an ice-water bath at 0-5°C for 2 hours, filter under reduced pressure, rinse the filter cake with 200 g of ethyl acetate, and dry the solid under va...
Embodiment 2
[0031] The preparation of embodiment 2 bazedoxifene acetate
[0032] The reaction formula is as follows:
[0033]
[0034] Add 600g of ethyl acetate, 180g of the compound shown in Formula A, 360g of ethanol, 45g of ammonium formate and 9g of 5% palladium-carbon catalyst in a 2000ml three-necked flask successively under stirring; heat up to 65°C and react for 16-28 hours until TLC shows that the raw materials and single The benzyl point disappears, (or the remaining single benzyle detected by HPLC≤0.1%). Filtrate under reduced pressure, rinse the filter cake with 900g of ethyl acetate, put the filtrate into a 5L separatory funnel, wash with 600g×3 water, add 25.0g of acetic acid to the organic layer while stirring in a 2L single-necked bottle, and solids precipitate out in about 10 minutes, then After stirring for 20 minutes, cool in an ice-water bath at 0-5°C for 2 hours, filter under reduced pressure, rinse the filter cake with 200 g of ethyl acetate, and dry the solid un...
Embodiment 3
[0035] The preparation of embodiment 3 bazedoxifene acetate
[0036] The reaction formula is as follows:
[0037]
[0038]Add 590g of ethyl acetate, 180g of the compound represented by formula A, 360g of ethanol, 58g of 1,3-cyclohexadiene and 9g of 5% palladium on carbon catalyst in a 2000ml three-necked flask with stirring; heat up to 65°C and react for 16 to 28 hours until TLC shows that the starting material and the monobenzyl compound disappear, (or the residual monobenzyl compound detected by HPLC≤0.1%). Filtrate under reduced pressure, rinse the filter cake with 500g of ethyl acetate, add 25.0g of acetic acid to the filtrate under stirring in a 2L single-necked bottle, and solids will precipitate out in about 10 minutes, then stir for 20 minutes, then cool in an ice-water bath at 0-5°C for 2 hours, reduce Filter under pressure, rinse the filter cake with 200 g of ethyl acetate, and dry the solid under vacuum at 45° C. for 2 hours. 130.8 g of off-white solid, namely ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com