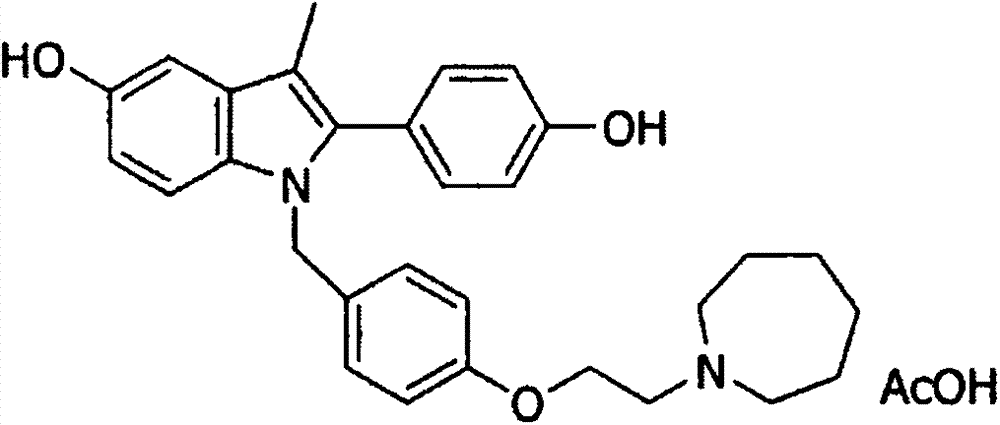
Bazedoxifene acetate sustained release preparation with excellent performance
The technology of bazedoxifene acetate and preparation is applied in the field of bazedoxifene acetate sustained-release preparation and its preparation technology, which can solve the problems such as no research report on sustained-release preparation, achieve lasting and stable blood concentration and simple preparation process , the effect of improving medication adherence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
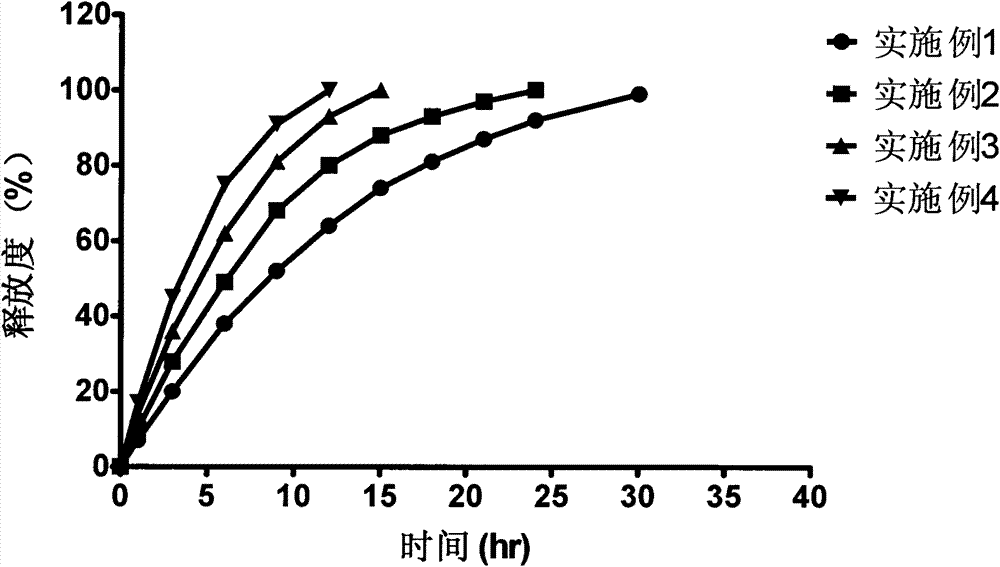
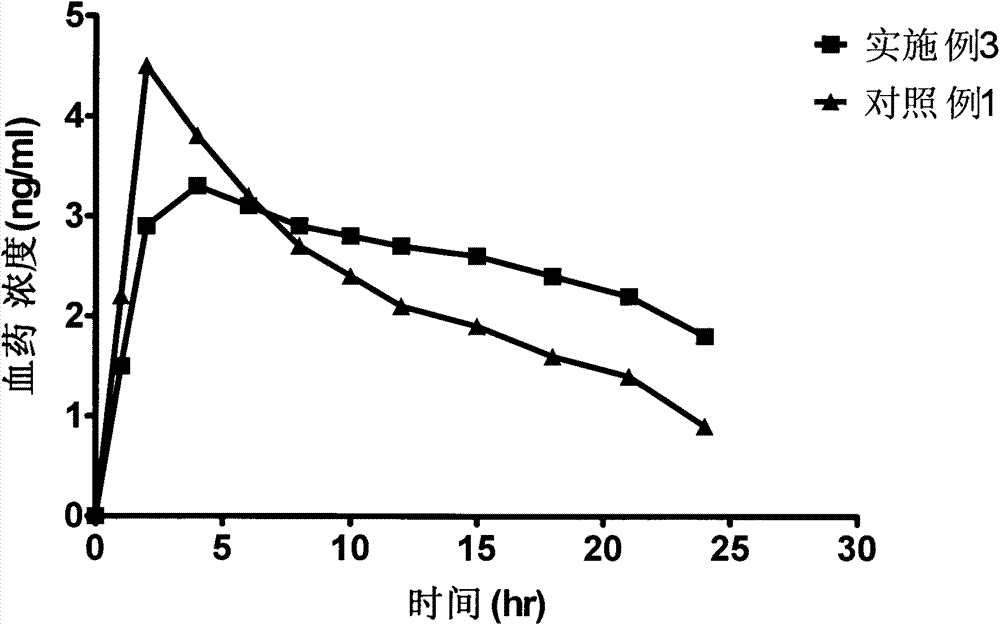
Image
Examples
Embodiment 1
[0028] Prescription: Dosage per 1000 capsules:
[0029] Bazedoxifene Acetate 2.26g
[0030] Vitamin E TPGS 5.0g
[0031] Stearic acid 42.74g
[0032] Specifications: main drug content 2.26mg / grain, pill weight 50mg / grain.
[0033] Process: Grind the raw material of bazedoxifene acetate and finely grind it to more than 150 meshes; weigh vitamin E TPGS and stearic acid according to the prescription; heat and melt the hydrophilic matrix, add bazedoxifene acetate and mix well, then add hydrophobic Mix the base of the dropping pills to form a suspension; keep the above eutectic solution at 80-85°C; In the methyl silicone oil, remove and drain the silicone oil, and wipe off the silicone oil attached to the surface of the dripping pill with medical gauze.
Embodiment 2
[0035] Prescription: Dosage per 1000 capsules:
[0036] Bazedoxifene Acetate 4.52g
[0037] Vitamin E TPGS 10.0g
[0038] Glyceryl monostearate 35.48g
[0039] Specifications: main drug content 4.52mg / grain, pill weight 50mg / grain.
[0040] Process: Grind and finely grind bazedoxifene acetate raw materials to more than 150 meshes; weigh vitamin E TPGS and monostearic acid glyceric acid according to the prescription; heat and melt the hydrophilic matrix, add bazedoxifene acetate and mix well Then add the hydrophobic dropping pill matrix and mix well to form a suspension; keep the above eutectic solution at 80-90°C; In the simethicone oil at room temperature, remove the silicone oil and wipe off the silicone oil attached to the surface of the dropping pills with medicinal gauze.
Embodiment 3
[0042] Prescription: Dosage per 1000 capsules:
[0043] Bazedoxifene Acetate 2.26g
[0044] Vitamin E TPGS 15.0g
[0045] Octadecanol 32.74g
[0046] Specifications: main drug content 2.26mg / grain, pill weight 50mg / grain.
[0047] Process: Grind and finely grind bazedoxifene acetate raw materials to more than 150 meshes; weigh vitamin E TPGS and stearyl alcohol according to the prescription; heat and melt the hydrophilic matrix, add bazedoxifene acetate and mix well, and then add hydrophobic Mix the base of the dropping pills to form a suspension; keep the above eutectic solution at 80-90°C; In the methyl silicone oil, remove and drain the silicone oil, and wipe off the silicone oil attached to the surface of the dripping pill with medical gauze.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com