Method for improving VEGF-receptor blocking selectivity of an anti-VEGF antibody
A technology of VEGF-R2 and VEGF-A121, applied in the field of antibodies, can solve problems such as insignificant inhibition, and achieve the effect of reducing side effects and improving safety characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Production of Human Anti-VEGF Antibody (Antibody VEGF-0089)
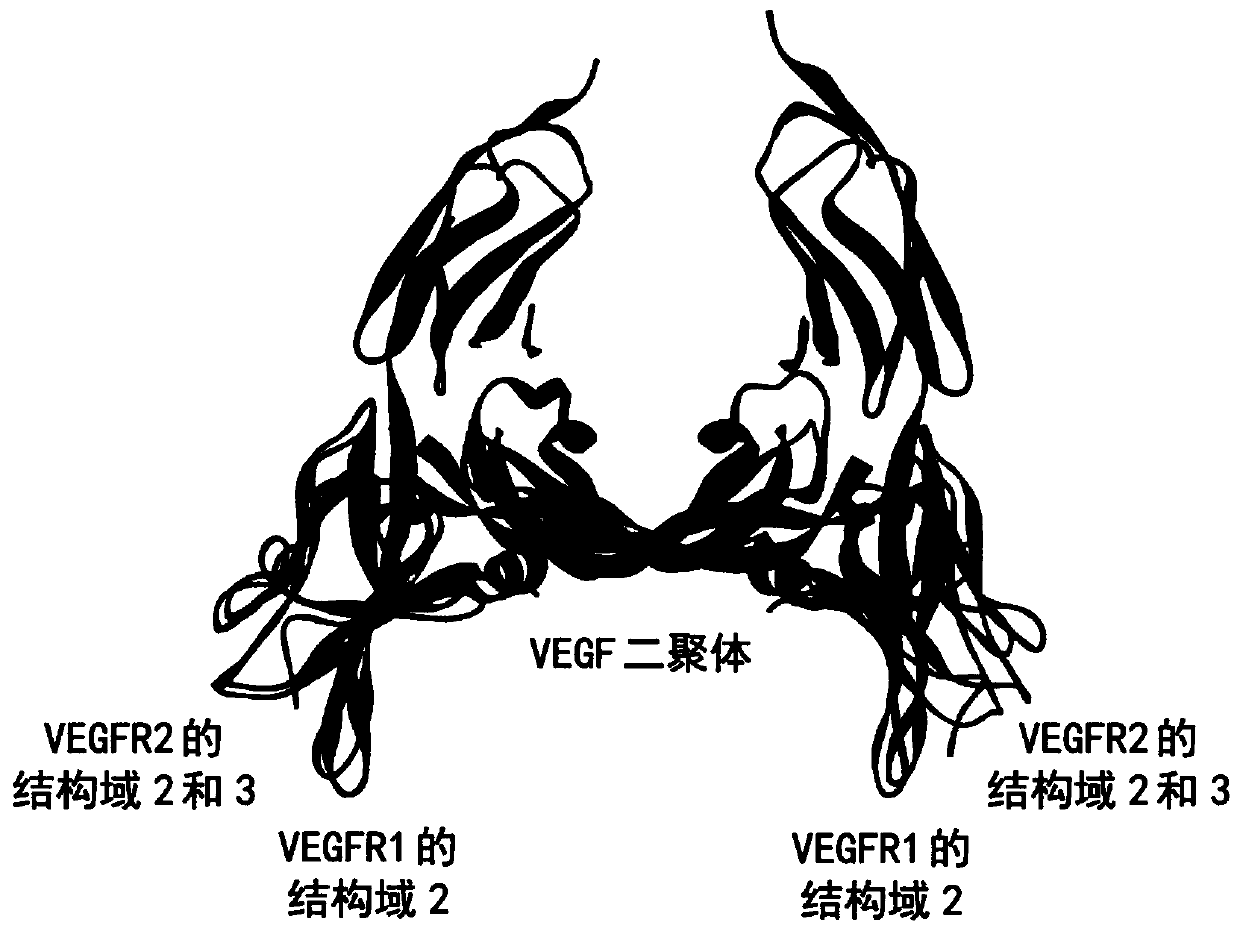
[0086] figure 1 Superposition of the crystal structure of the human VEGF dimer in complex with VEGF-R1 domain 2 and VEGF-R3 domains 2 and 3 is depicted. This overlay illustrates that both VEGF receptors bind to highly similar regions on the VEGF dimer, so antibodies that bind VEGF in the same manner without inhibiting VEGF binding to both receptors VEGF-R1 and VEGF-R2 appear to be highly challenging. Consistent with this, among the various anti-VEGF antibodies known in the art, only a few antibodies have been reported to selectively block the binding of VEGF to VEGF-R2 but not the binding of VEGF to VEGF-R1.
[0087] The antibody VEGF-0089 described herein was derived from Roche's proprietary transgenic rabbits expressing a humanized antibody repertoire following immunization with VEGF-derived antigens. Transgenic rabbits comprising human immunoglobulin loci are reported in WO2000 / 46251, WO2002 / 12437, WO20...
Embodiment 2
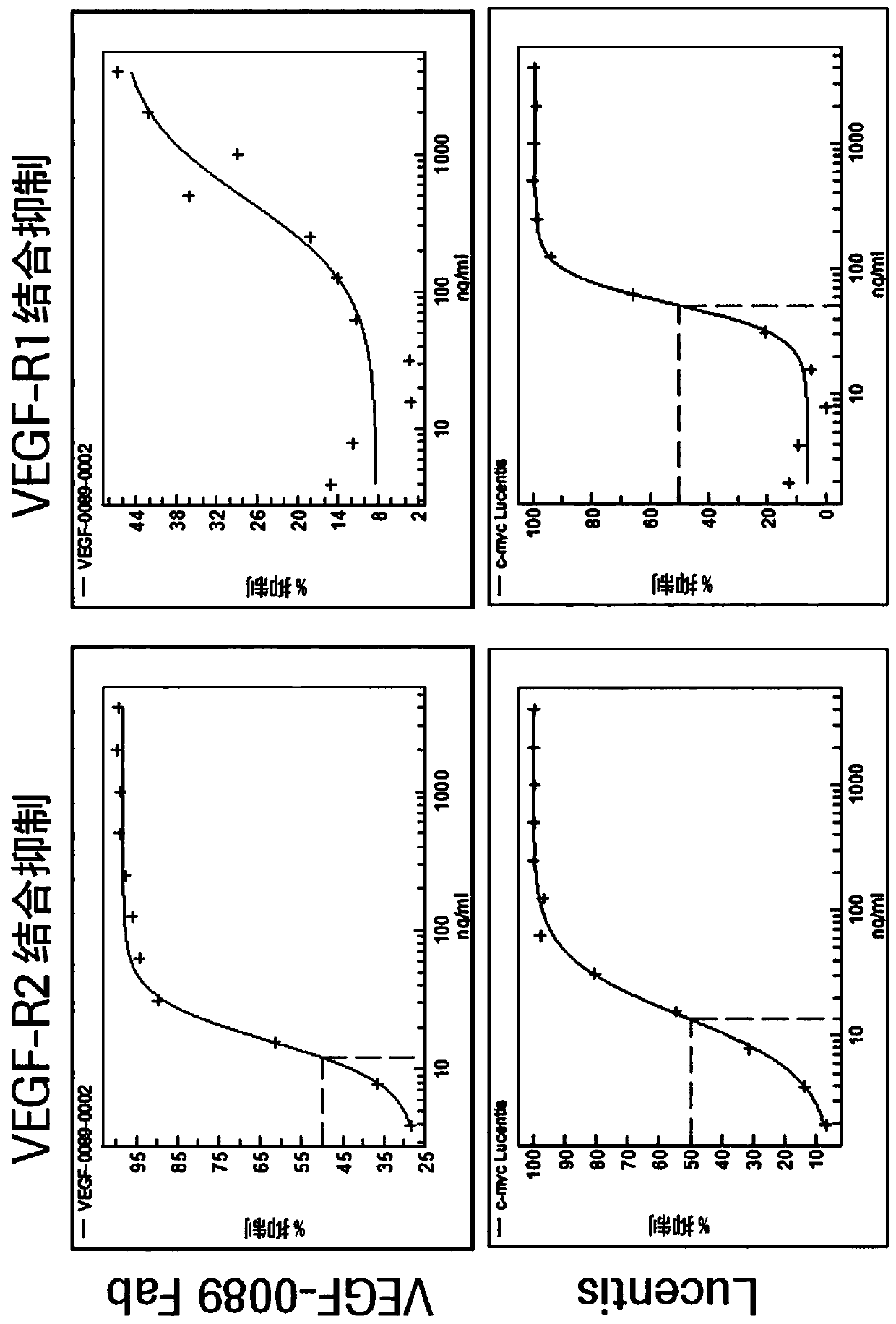
[0100] Characterization of the generated human anti-VEGF antibody (antibody VEGF-0089)
[0101] VEGF binding of antibody VEGF-0089 Fab fragment was assessed by surface plasmon resonance (SPR) as described below.
[0102] Determination of Antibody Binding Affinity by Surface Plasmon Resonance (SPR)
[0103] An anti-His capture antibody (GE Healthcare 28995056) was immobilized to a Series S sensor chip C1 (GE Healthcare 29104990) using standard amine coupling chemistry, resulting in a surface density of 500-1000 resonance units (RU). As running and dilution buffers, HBS-P+ (10 mM HEPES, 150 mg / mL NaCl, pH 7.4, 0.05% surfactant P20) was used, and the measurement temperatures were set at 25°C and 37°C, respectively. hVEGF-A121 was captured to the surface resulting in capture levels of 5-35RU. A dilution series (0.37-30 nM) of anti-VEGF antibody was injected for 120 s and dissociation was monitored for at least 600 s at a flow rate of 30 μl / min. The surface was regenerated by in...
Embodiment 3
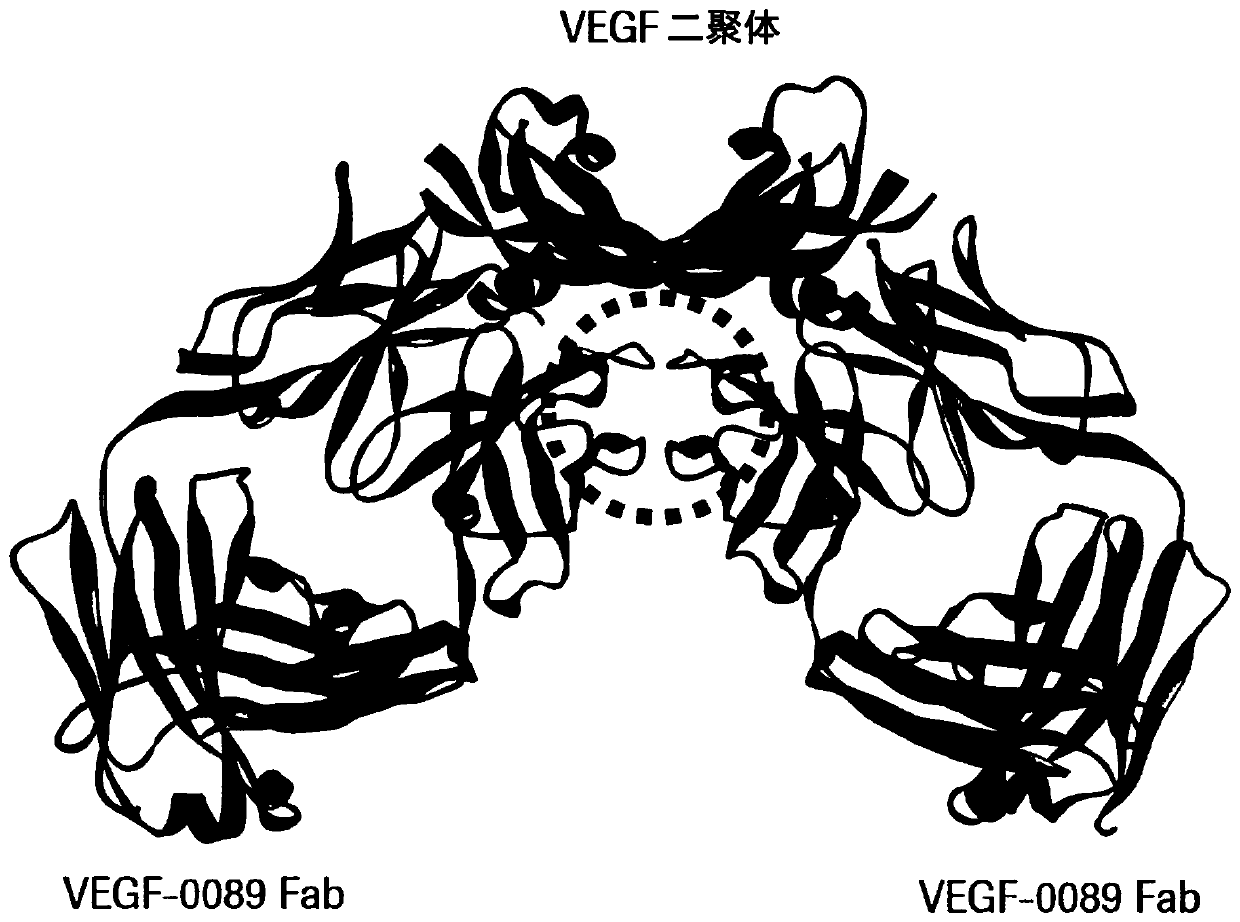
[0111] X-ray crystallography and epitope determination of antibody VEGF-0089 in complex with VEGF-dimer
[0112] The crystal structure of the VEGF-0089 Fab fragment described above was analyzed according to standard methods known in the art.
[0113] X-ray crystallography of VEGF-0089 Fab fragment complexed with VEGF-A121 was performed as follows:
[0114] Complex formation and purification of the dimeric complex VEGF-A121-VEGF-0089 Fab. For complex formation, VEGF-0089 Fab fragment and human VEGF-A121 (Peprotech) were mixed in a molar ratio of 1.1:1. After overnight incubation at 4°C for 16 hours, the complex was purified by gel filtration chromatography on a Superdex200 (16 / 600) column in 20 mM MES, 150 mM NaCl, pH 6.5. Fractions containing the dimer complex were pooled and concentrated to 1.44 mg / ml.
[0115] Crystallization of the dimeric VEGF-A121-VEGF-0089 Fab complex. Initial crystallization experiments were performed at 21°C in a sitting drop vapor diffusion appara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com