Study and application of release technique of effective compound group of traditional Chinese Medical prescription
A compound and traditional Chinese medicine technology, applied in the field of medicine, can solve the problems of weak research foundation, insufficient pharmacokinetic parameters for evaluating clinical efficacy, and inability to represent drug absorption, action characteristics and laws, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Tablet Core Prescription Danshen Extract 15%
[0068] Panax notoginseng powder 30%
[0069] Borneol 2%
[0071] PEO(WSR N~750) 30%
[0072] Poloxamer 5%
[0074] Coating film prescription: cellulose acetate 3g
[0075] PEG 4000 5g
[0076] Acetone 100ml
[0077] Tablet core preparation: mix Danshen extract, Panax notoginseng extract, borneol and various excipients passed through a 100-mesh sieve evenly, add an appropriate amount of magnesium stearate as a lubricant, and directly compress the whole powder after mixing to obtain tablets core. Preparation of coating solution: dissolve the prescribed amount of cellulose acetate and PEG 4000 in acetone to obtain the coating solution.
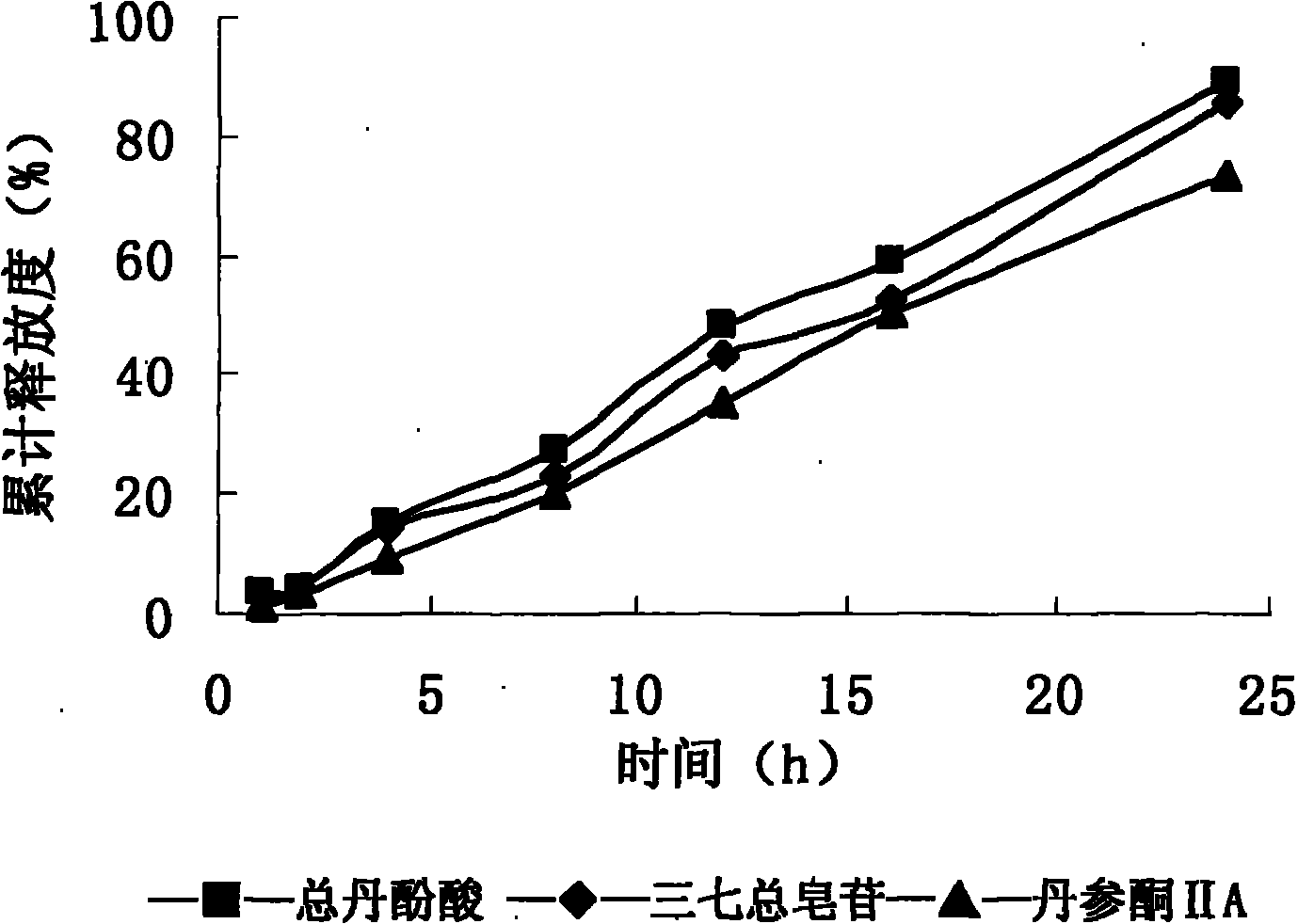
[0078] Coating: Put the tablet core in a coating pan, spray the coating solution, and dry at 40°C for 48 hours after coating. A...
Embodiment 2
[0081] Tablet Core Prescription: Danshen Extract 15%
[0082] Panax notoginseng extract 30%
[0083] Borneol 2%
[0085] PEO (WSR N~750) 35%
[0087] Coating film prescription: cellulose acetate 3g
[0088]PEG 4000 5g
[0089] Acetone 100ml
[0090] Tablet core preparation: mix Danshen extract, Panax notoginseng extract, borneol and various excipients passed through a 100-mesh sieve evenly, add an appropriate amount of magnesium stearate as a lubricant, and directly compress the whole powder after mixing to obtain tablets core.
[0091] Coating solution preparation: Dissolve 4 g of cellulose acetate and 3 g of potassium chloride in 100 ml of acetone to obtain the coating solution.
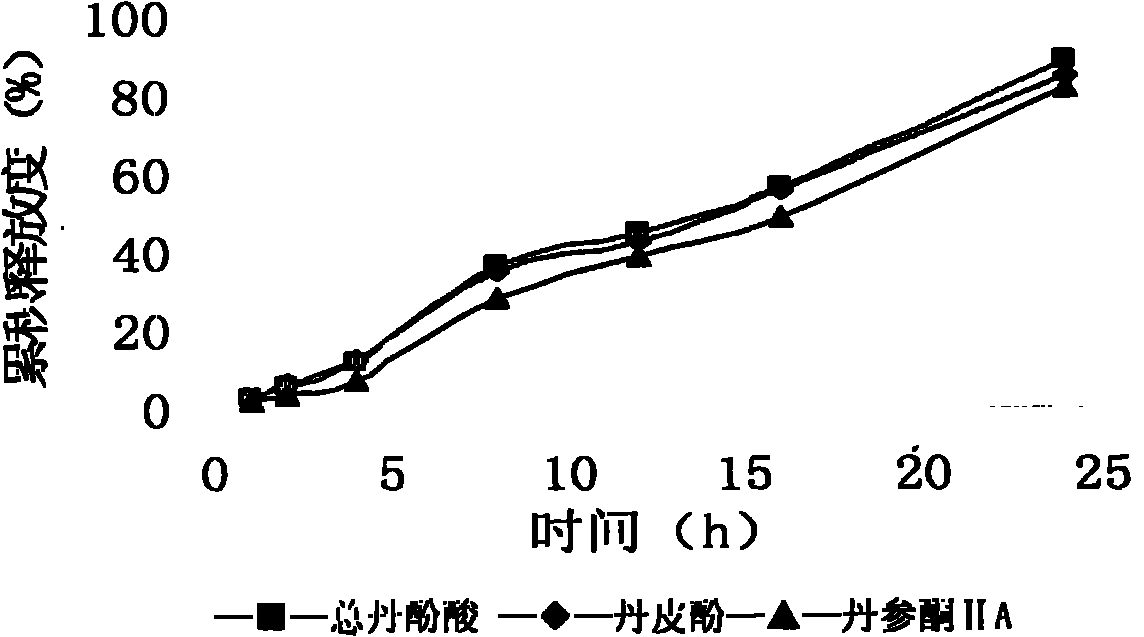
[0092] Coating: Put the tablet core in a coating pan, spray the coating solution, and dry at 40°C for 48 hours after coating. The coating weight ...
Embodiment 3
[0095] Tablet core prescription: Liuwei Dihuang Extract 38%
[0097] Sodium bicarbonate 5%
[0098] PEO (WSR N~750 30%
[0099] Poloxamer 5%
[0101] Coating film prescription: cellulose acetate 3g
[0102] PEG 4000 6g
[0103] Acetone 100ml
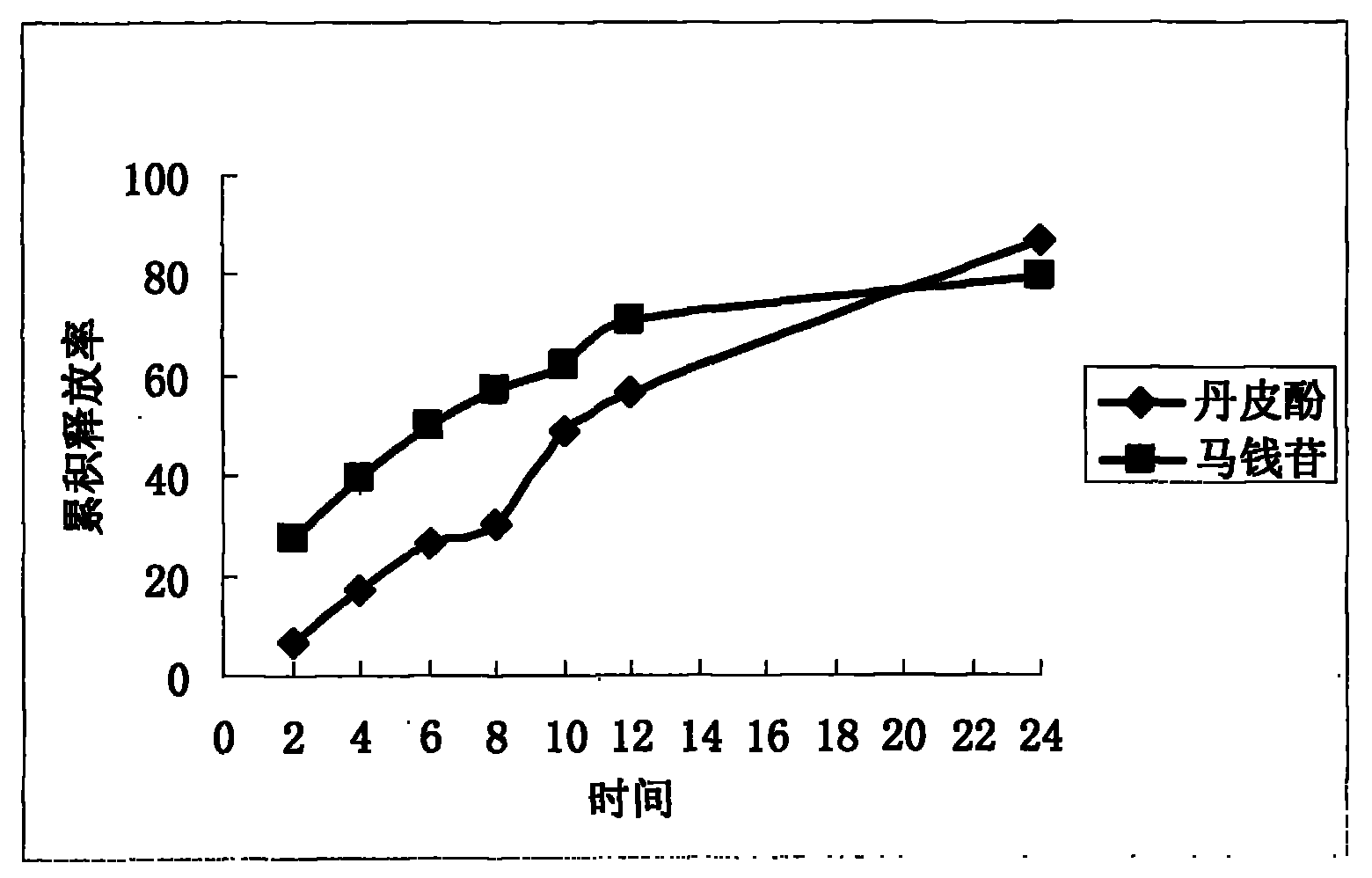
[0104] Preparation process The Liuwei Dihuang extract, sodium chloride, and sodium bicarbonate were pulverized and passed through a 100-mesh sieve, and each raw and auxiliary material was weighed according to the prescription amount, and the whole powder was directly compressed after mixing to obtain a tablet core; cellulose acetate, Dissolve PEG-4000 (dissolve first with a small amount of water) in acetone solvent, stir until the cellulose acetate is completely dissolved to obtain the coating solution; place the tablet core in the coating pan for coating, and control the temperature of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com