Paliperidone derivative low-dose tablet and preparation method thereof
A technology of paliperidone and derivatives, applied in the field of low-dose tablets of paliperidone derivatives, to achieve the effect of reducing adverse reactions and side effects, improving therapeutic effect, and reducing irritation and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
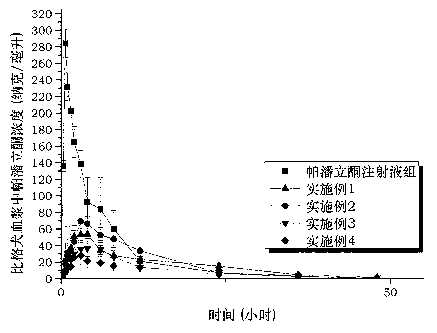
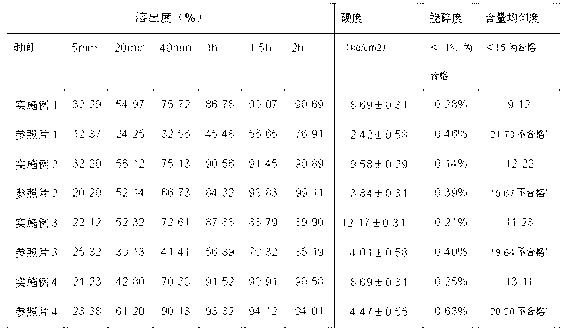
Image
Examples
Embodiment 1
[0031] Example 1 N-pentoxy paliperidone tablets with a drug loading of 1.5%
[0032] Prescription: n-pentoxy paliperidone 1.5g, spray-dried lactose monohydrate (Flowlac? 100) 83.5g, hydroxypropylmethylcellulose (HPMC K100LV CR) 15g, magnesium stearate 1g, 500 tablets in total.
[0033] Weigh n-pentyloxy paliperidone, Flowlac? 100, HPMC K100LV CR according to the prescription, and use a three-dimensional mixer to mix for 10 minutes to achieve a uniform mixing state; transfer the material mixture to a small mixer at a speed of 25 rpm, and spray the wetting agent for 70 % ethanol 35-40mL can be used to prepare soft materials in a suitable state; use a wet granulator with a 1.6mm sieve to make wet granules, and the speed is 90rpm, until no particles fall from the sieve, and there is no residual unsifted soft material on the sieve. The granulation is completed; dry in a blast drying oven at 45°C for 30 minutes, and keep stirring during the period, until the material is dry, evenly ...
Embodiment 2
[0035] Example 2 Isobutoxy Paliperidone Tablets with 1.5% Drug Loading
[0036] Prescription: Isobutoxy paliperidone 1.5g, spray-dried lactose monohydrate (SuperTab? 11SD) 83.5g, HPMC K100LV CR 15g, magnesium stearate 1g, 500 tablets in total
[0037] Weigh isobutoxy paliperidone, SuperTab? 11SD, HPMC K100LV CR according to the prescription, and use a three-dimensional mixer to mix for 10 minutes to achieve a uniform mixing state; transfer the material mixture to a small mixer at a speed of 25 rpm, and spray the wetting agent for 70 % ethanol 35-40mL can be used to prepare soft materials in a suitable state; use a wet granulator with a 1.6mm sieve to make wet granules, and the rotation speed is 90rpm, until no particles fall from the sieve, and there is no residual soft material that has not been sieved on the sieve. After the granulation is completed, the time required is about 15 minutes; dry in a blast drying oven at 45°C for 30 minutes, and keep stirring during the period,...
Embodiment 3
[0039] Example 3 Isobutoxy Paliperidone Tablets with 0.75% Drug Loading
[0040] Prescription: 3.75g isobutoxy paliperidone, 421.25g spray-dried lactose monohydrate (SuperTab? 11SD), 75g hypromellose, 5g magnesium stearate, 1000 tablets in total
[0041] Weigh isobutoxy paliperidone, SuperTab? 11SD, and HPMC K100LV CR according to the prescription, and mix them with a three-dimensional mixer for 15 minutes to achieve uniform mixing; transfer the material mixture to a small mixer at a speed of 25 rpm, and spray the wetting agent for 70 % ethanol is about 175-200mL to prepare soft materials in a suitable state; use a wet granulator with a 1.0mm sieve to make wet granules at a speed of 90rpm, until no particles fall from the sieve, and there is no remaining unsifted soft material on the sieve. After the granulation is completed, the granulation time is about 20 minutes; dry in a blast drying oven at 45°C for 40 minutes, and keep stirring during the period, until the material is d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com