A kind of acyclovir pharmaceutical composition
A composition and drug technology, used in drug delivery, active ingredients of heterocyclic compounds, pharmaceutical formulations, etc., can solve the problems of large dosage of sustained-release materials, sudden drug release, poor release consistency, etc. Stable and less adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
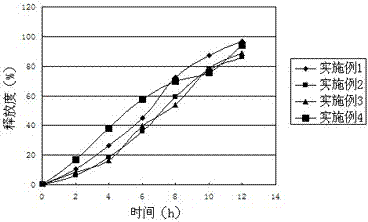
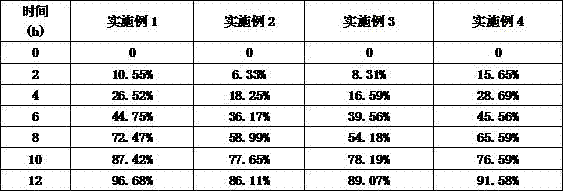
Embodiment 1
[0030] Acyclovir 44%
[0031] Ethyl Cellulose 30%
[0032] Sodium chloride 2%
[0033] Lactose 13%
[0034] Tragacanth Gum 10%
[0035] Talc 1%
Embodiment 2
[0037] Aciclovir 40%
[0038] Ethylcellulose 31%
[0039] Sodium chloride 2%
[0040] Lactose 13%
[0041] Tragacanth Gum 12%
[0042] Talc 1%
Embodiment 3
[0044] Aciclovir 45%
[0045] Ethylcellulose 27%
[0046] Sodium chloride 3%
[0047] Lactose 10%
[0048] Tragacanth Gum 13%
[0049] Talc 2%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com