Acyclovir pharmaceutical composition
A composition and drug technology, applied in the directions of drug delivery, active ingredients of heterocyclic compounds, pharmaceutical formulations, etc., can solve the problems of large dosage of sustained-release materials, poor release consistency, drug burst release, etc. Stable and less adverse effects
Active Publication Date: 2015-06-03
KAMP PHARMA
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The above-mentioned patented process adopts ordinary wet granulation, and the amount of sustained-release materials is relatively large; the release consistency is poor, which may easily cause sudden or uneven drug release
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
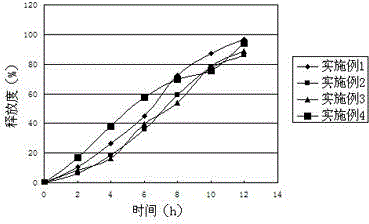
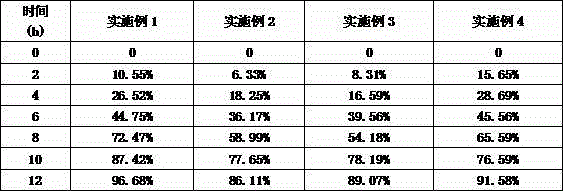
Embodiment 1
[0029] Acyclovir 44%
[0030] Ethyl Cellulose 30%
[0032] Lactose 13%
[0033] Tragacanth Gum 10%
[0034] Talc 1%
Embodiment 2
[0036] Acyclovir 40%
[0037] Ethylcellulose 31%
[0039] Lactose 13%
[0040] Tragacanth Gum 12%
[0041] Talc 1%
Embodiment 3
[0043] Aciclovir 45%
[0044] Ethylcellulose 27%
[0046] Lactose 10%
[0047] Tragacanth Gum 13%
[0048] Talc 2%
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to the field of medicine preparation, and in particular relates to an acyclovir pharmaceutical composition and a preparation method thereof. Common wet granulation is adopted in the prior art; the amount of a sustained-release material is relatively high; the release consistency is poor; and sudden release or uneven release is easily caused. The invention provides an acyclovir sustained-release tablet which is relatively high in medicine release stability and medication safety. According to the technical scheme provided by the invention, the acyclovir pharmaceutical composition comprises a mixture composed of acyclovir, a framework material, electrolyte, saccharides, hydrophilic gel and a lubricant. The acyclovir pharmaceutical composition is stable in sustained-release rate, and is a first-order speed; the peak and valley phenomena caused after multi-dose administration of a general preparation can be overcome; the medication frequency is reduced; the stimulation to gastrointestinal tracts is greatly reduced; and adverse reaction is reduced.
Description
technical field [0001] The invention relates to the field of pharmaceutical production, in particular to an acyclovir pharmaceutical composition and a preparation method thereof. Background technique [0002] Acyclovir, also known as acyclovir (ACV for short), is a nucleoside antiviral drug with a chemical name of 9-(2-hydroxyethoxymethyl)guanine. In 1981, Wellcome Company first listed this product in the UK, trade name: Zovirax. In 1982, the FDA approved this product to be marketed in the United States. At present, aciclovir is one of the main medicines sold worldwide. Acyclovir is the homotype of acyclic deoxyguanosine, which is a component of DNA. Acyclovir is similar in structure, except that the cyclic sugar structure is replaced by an acyclic side chain. It is mainly used for various infections caused by herpes simplex virus, and can be used for initial or recurrent skin, mucous membrane, external genital infection and HSV infection in immunocompromised persons. It...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/22A61K31/522A61K47/38A61K47/34A61K47/32A61P31/22
Inventor 曾培安吴健民张浩贺莲
Owner KAMP PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com