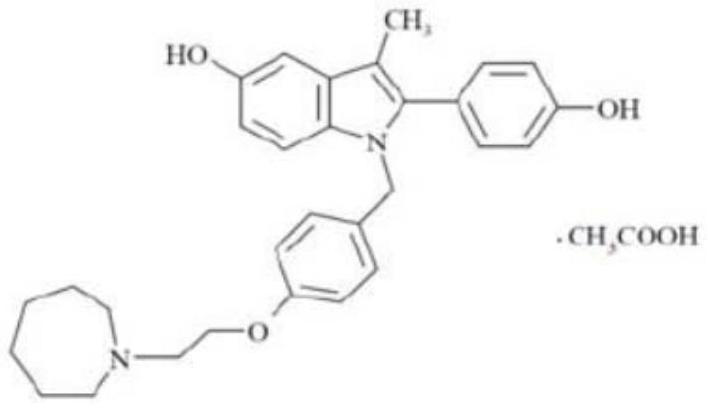
Bazedoxifene acetate composition and bazedoxifene acetate film-coated tablet preparation method
A technology of bazedoxifene acetate and its composition, which is applied in the field of drug preparation, can solve problems such as cumbersome process, and achieve the effects of simple process, good stability, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
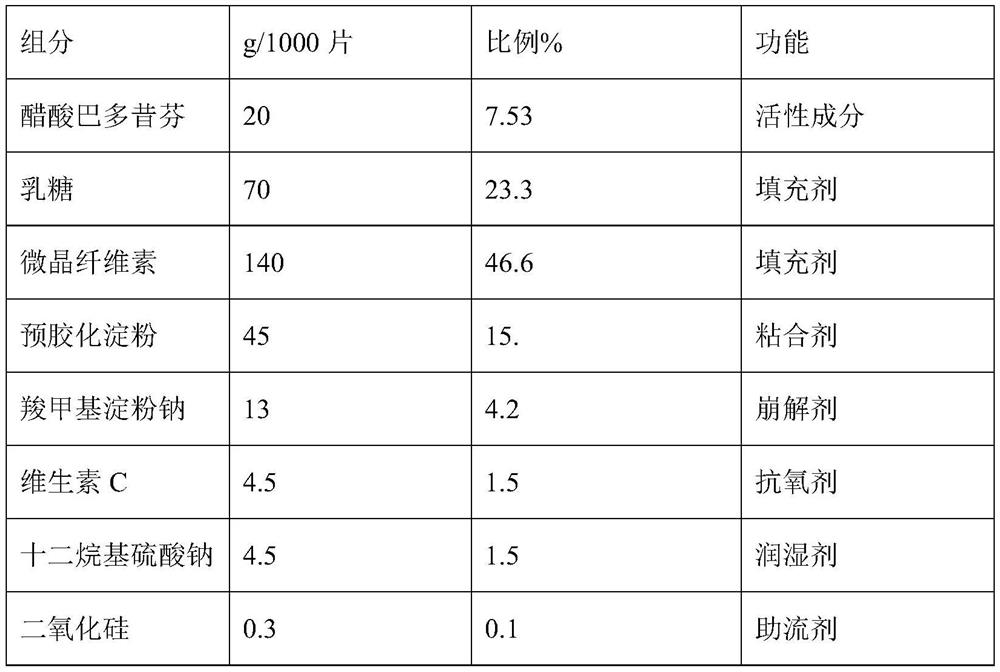
[0024] Embodiment 1: A kind of prescription composition of bazedoxifene acetate tablet
[0025]
[0026]
Embodiment 2
[0027] Embodiment 2: A kind of prescription composition of bazedoxifene acetate tablet
[0028] components g / 1000 tablets Proportion% Function bazedoxifene acetate 22.6 7.53 active ingredient lactose 105 35 filler microcrystalline cellulose 105 35 filler pregelatinized starch 44.63 14.87 Adhesive Sodium carboxymethyl starch 12 4 disintegrant Vitamin C 4.5 1.5 antioxidant Sodium dodecyl sulfate 4.5 1.5 D silica 0.3 0.1 Glidant Magnesium stearate 1.5 0.5 lubricant
Embodiment 3
[0029] Embodiment 3: A kind of prescription composition of bazedoxifene acetate tablet
[0030]
[0031]
[0032] The preparation process description of bazedoxifene acetate tablet:
[0033] Sodium lauryl sulfate and purified water are prepared into a solution with a concentration of about 10% for later use.
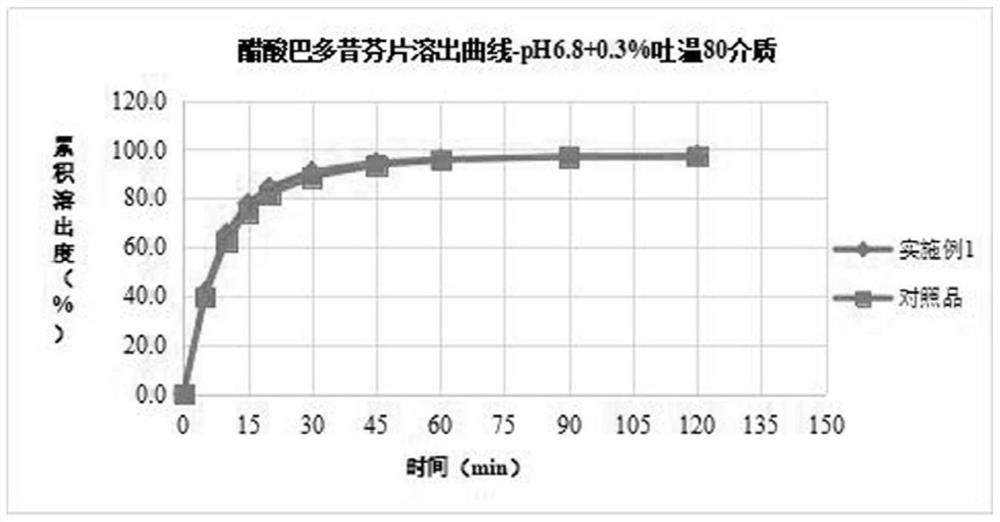
[0034] The bazedoxifene acetate, filler, binder, disintegrant, and antioxidant are weighed and mixed together, and after mixing, a wetting agent solution is added to carry out wet granulation or fluidized bed granulation process. After granulation, dry at 50-60°C to control the water content to 1-4%, pass through a 40-mesh sieve for granulation. Add the rest of the materials, mix them, compress into tablets and coat them. Comparative example: in vitro release test of prepared samples
[0035] Control sample: bazedoxifene acetate tablets (specification 20 mg) with the trade name Viviant, manufactured by PfizerJapan Inc. / ファイザー Co., Ltd., batch number CJ6968, packa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com