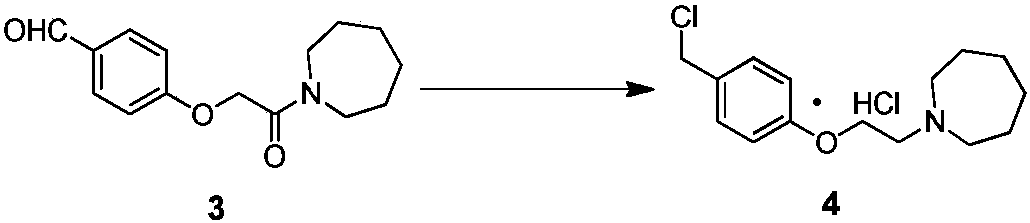Industrial production method for bazedoxifene acetate
A technology of bazedoxifene acetate and a production method, applied in the directions of organic chemistry, carboxylate preparation, etc., can solve problems such as difficult post-processing purification, achieve high economic value and social benefits, easy availability of cost, reduced complexity and low cost. The effect of manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
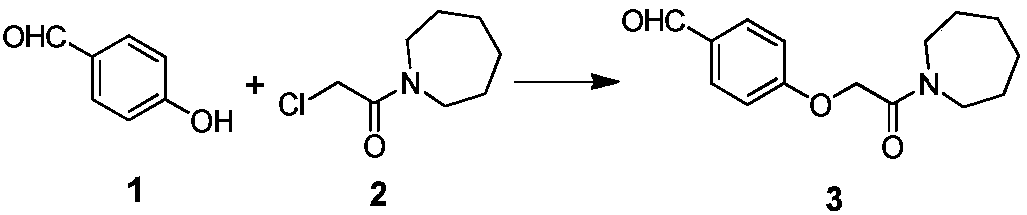
[0041]The specific synthetic operation of compound 3 is as follows:
[0042] In a 1000L reactor, add 200kg of DMF, add 17kg of compound 1 and 30kg of compound 2 at room temperature, raise the temperature to 40°C, add 600kg of purified water after the reaction is complete, separate the organic phase, concentrate and spin dry, and obtain 31.2kg of light brown Solid, yield: 93.5%, HPLC purity: 95.1%.
[0043] Table 1 Compound 3 yield quality compared with literature method
[0044]
[0045] [1] The literature method is CN 102690225B Example 1
Embodiment 2
[0047] The specific synthetic operation of compound 4 is as follows:
[0048] In a 500L reactor, add 130kg of boron trifluoride ether solution, 30kg of compound 3 prepared in Example 1, and 6.4kg of sodium borohydride, and react at 15°C for 2 hours. After the reaction, quench with 25kg of saturated ammonium chloride solution, and then Add 80kg of 5% sodium hydroxide solution, filter and dry to obtain off-white solid. The reaction kettle was cooled to 5°C, and 75kg of thionyl chloride was added dropwise. After the reaction, 100kg of acetone was added to stir, filtered, and dried to obtain 27.8kg of light brown solid, yield: 85.1%, HPLC purity: 96.3%.
[0049] Table 2 Compound 4 yield quality compared with literature method
[0050] Example
[0051] [1] The literature method is CN 104151265 B embodiment 1~4 method.
Embodiment 3
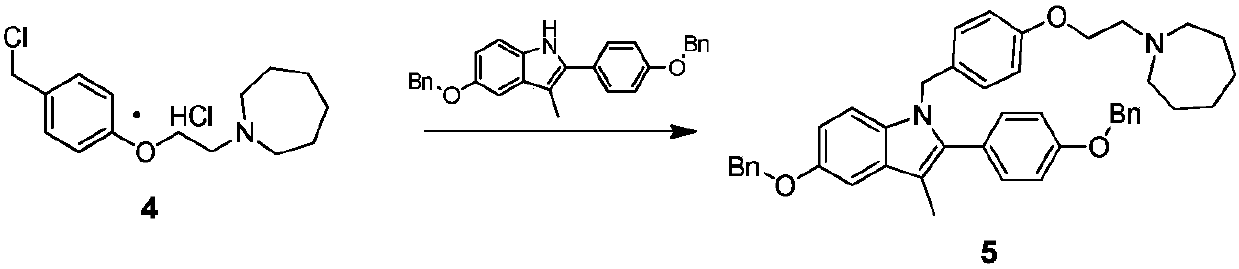
[0053] The specific synthetic operation of compound 5 is as follows:
[0054] Add 90kg of DMF to a 1000L reactor, add 30kg of 5-(benzyloxy)-2-(4-(benzyloxy)phenyl)-3-methyl-1H-indole at room temperature and stir until dissolved, add 60kg of sodium hydroxide and 12kg of water were activated for 1h, then the DMF solution of compound 4 prepared in Example 2 was added (24kg of compound 4 was dissolved in 210kg of DMF), and the reaction was carried out at room temperature for 3h. After the reaction was completed, 600kg of purified water was added, stirred and crystallized at room temperature for 8h , filtered and dried to obtain 38.5kg off-white solid, yield: 82.7%, HPLC purity 95.3%.
[0055] Add 90kg of DMSO to a 1000L reactor, add 30kg of 5-(benzyloxy)-2-(4-(benzyloxy)phenyl)-3-methyl-1H-indole at room temperature and stir until dissolved, add 50kg of solid sodium hydroxide and 12kg of water were activated for 1h, then the DMSO solution of compound 4 was added (24kg of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com