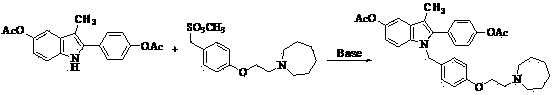Preparation method of bazedoxifene acetate and key intermediate thereof
A technology of bazedoxifene acetate and intermediates, applied in the direction of organic chemistry and the like, can solve problems such as hidden dangers, existing operation safety, difficulty in industrialization amplification, etc., and achieve the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
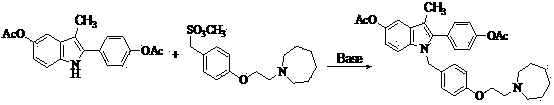
[0028] Example 1 : 1-[4-(2-azepan-1-yl-ethoxy)-benzyl]-2-(4-acetoxy-phenyl)-3-methyl-5-acetoxy- 1 H - Preparation of indole (compound III).
[0029] I II III
[0030] In a 1000ml three-neck flask, add 32.3g (0.1mol) of compound I, 36.1g (0.11mol) of compound II, 500ml of toluene, stir, heat to 75°C for 4 hours, TLC spotting test shows that the reaction is complete, distill and remove the solvent , to obtain a residue, add 100ml of water, extract with ethyl acetate (200ml×3), dry over anhydrous sodium sulfate, filter off the desiccant, and distill under reduced pressure to obtain a light white solid 51.2, yield: 92.5%.
[0031] MS (+1): 555.2.
[0032] 1 HNMR: δ (ppm, CDCl 3 ), 7.45-7.46(d, 2H, Ar-H), 7.12-7.13(d, 2H, Ar-H), 7.06-7.10(m, 3H, Ar-H), 6.95-6.96(d, 2H, Ar-H) -H), 6.73-6.75(d, 2H, Ar-H), 5.11(s, 2H, CH 2 ), 4.10-4.12 (t, 2H, CH 2 ), 2.78-2.79(t, 2H, CH 2 ), 2.36-2.38(t, 4H, CH 2 ×2), 2.09(s, 3H, CH 3 ), 2.07(s, 3H, CH 3 ), 1.39-...
Embodiment 2
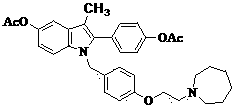
[0034] Example 2 : Preparation of Bazedoxifene Acetate (Compound TM)
[0035]
[0036] IIITM
[0037] In a 500ml three-neck flask, add 27.2g (0.05mmol) of compound III and 200ml of 1N (0.20mol) sodium hydroxide solution, stir, heat up to 55°C for 2 hours, TLC spotting test shows that the reaction is complete, cool to room temperature, and adjust the pH To neutrality, a solid was precipitated, and vacuum-dried to obtain 20.6g of a white solid, yield: 87.6%. The obtained solid was placed in a 500ml three-neck flask, 250ml of acetone was added, 20ml of acetic acid was added, heated to 60°C, and reacted for 3h. According to TLC detection, the reaction was completed, cooled, evaporated to remove the solvent and excess acetic acid, washed with pure water (100ml×3), and dried to obtain 20.1g of a white solid with a yield of 87.0%.
[0038] Free base MS (+1): 471.
[0039] HNMR: δ (ppm, CDCl 3 ), 7.46-7.48(d, 2H, Ar-H), 7.15-7.17(d, 2H, Ar-H), 6.95-6.96(d, 2H, Ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com