A kind of crystal form of valbenazine di-p-toluenesulfonate and its preparation method and use
A technology of p-toluenesulfonate and crystal form, applied in the field of medicinal chemistry, can solve the problems of low yield and large loss of raw materials, and achieve the effects of low adhesion, excellent adhesion and improved product appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The preparation method of embodiment 1 crystal form CSIII
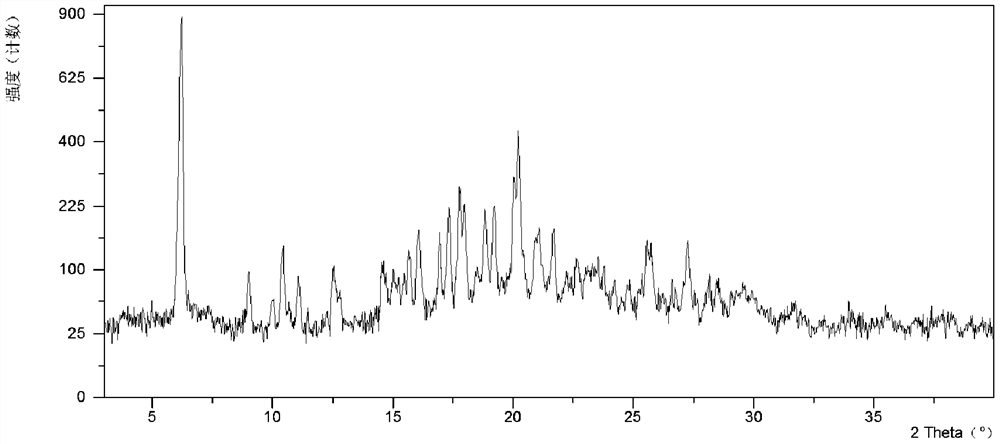
[0117] Weigh 432.1 mg of free base in a glass vial, add 390.8 mg of p-toluenesulfonic acid, then add 1 mL of 2-MeTHF into the vial, stir at room temperature for 3 minutes, add 1 mL of 2-MeTHF and 200 μL of water, stirred at -20°C, and the solid was separated to obtain the crystal form CSIII. Form CSIII is a co-solvate of 2-MeTHF and water, its XRPD pattern is as follows figure 1 shown.
Embodiment 2
[0118] The preparation method of embodiment 2 crystal form A
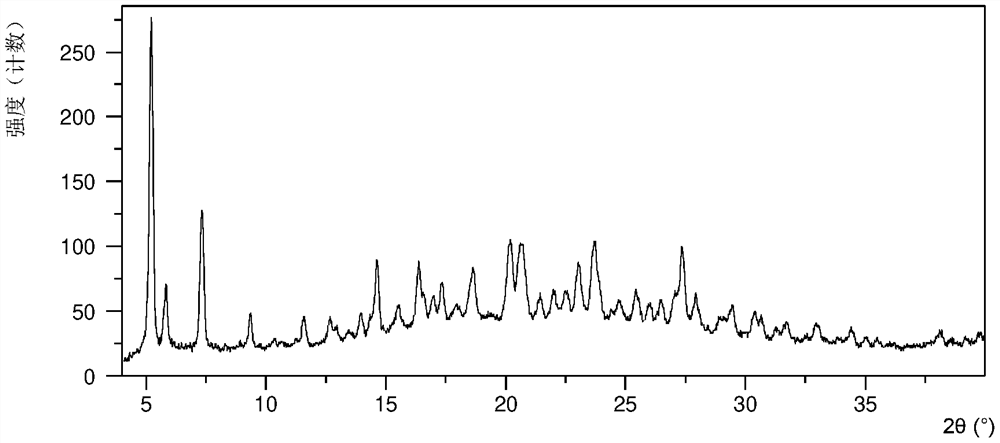
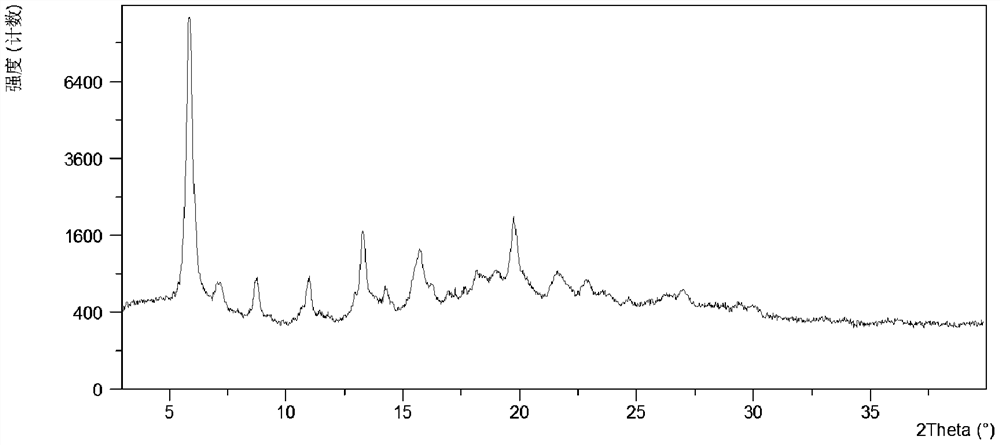
[0119] Put 46.2mg of crystal form CSIII in 2.3mL of anisole solvent, suspend and stir at 4°C, and separate the solid. At this time, the obtained solid is crystal form N4, and its XRPD pattern is as follows figure 2 shown. Form N4 was vacuum-dried at room temperature to obtain a white solid. After testing, the obtained crystalline solid is the crystal form A of the present invention, and its X-ray powder diffraction data are shown in Table 1, and its XRPD pattern is as follows image 3 .
[0120] Table 1
[0121] Diffraction angle 2θ d value strength% 5.88 15.03 100.00 7.06 12.52 3.97 8.68 10.18 4.82 10.98 8.05 5.50 13.29 6.66 14.80 14.27 6.21 3.81 15.75 5.63 10.38 16.26 5.45 4.06 16.96 5.23 2.53 18.15 4.89 5.91 19.00 4.67 5.70 19.76 4.49 17.66 21.58 4.12 4.90 22.88 3.89 3.61 26.98 3.30 2.41 29.40 3.04 ...
Embodiment 3
[0122] The preparation method of embodiment 3 crystal form A
[0123] Weigh 500.1mg of free base and 476.1mg of p-toluenesulfonic acid into a 20mL glass vial, add 4.0mL of 2-MeTHF, stir at -20°C for about 1.5h, continue to add 6.0mL of 2-MeTHF, stir After about 1.5 hours, centrifuge, and dry in vacuum at room temperature for about 1.5 hours to obtain a solid.
[0124] Transfer the solid to a 100mL vial, add about 45mL of anisole solvent and stir at -20°C for a period of time, then add about 0.5mL of crystal form N4 seed crystal suspension and continue stirring. After filtering under the condition and vacuum drying at room temperature overnight, 795.8 mg of crystalline form A was obtained (yield after deducting the seed crystal: 84%). Its XRPD pattern is as follows Figure 4 As shown, the XRPD data are shown in Table 2. TGA as Figure 5 As shown, there is about 2.61% mass loss when it is heated to 150°C. DSC such as Figure 6 As shown, it appears an endothermic peak aro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com