Metformin compound pharmaceutical composition and preparation method thereof
A technology of metformin hydrochloride and a composition, applied in the field of pharmacy, can solve the problems of increased difficulty in industrialization of three-layer tablets, many control points, poor patient compliance, etc., achieves stable and controllable product quality, and solves poor compressibility and production costs. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
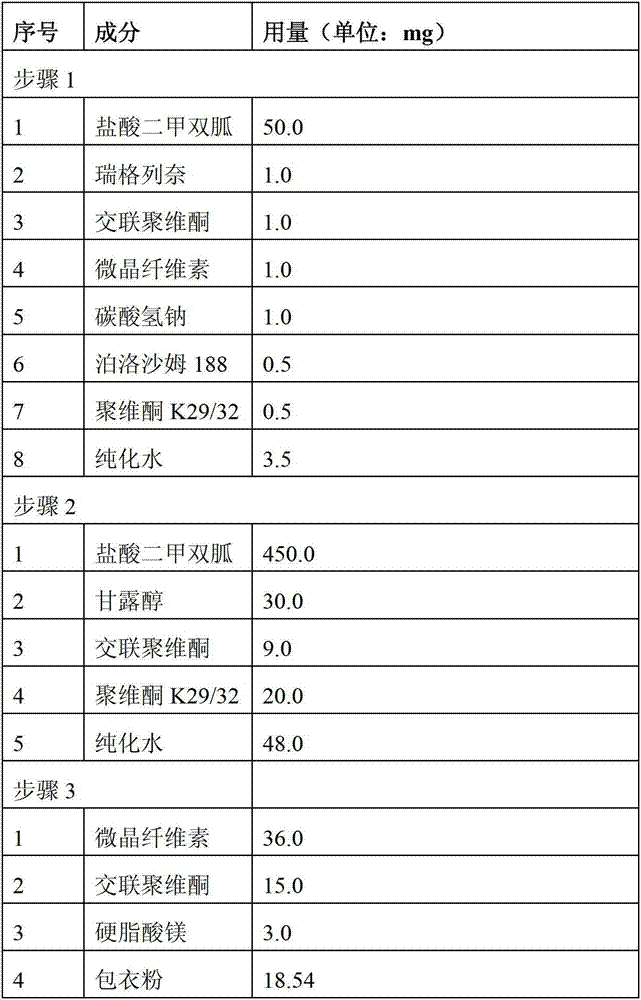
[0048] Preparation of single-dose pharmaceutical composition of repaglinide 1.0 mg and metformin hydrochloride 500 mg
[0049] Prepare according to the ingredients and dosage of each step in the table below:
[0050]
[0051] Step 1: mix repaglinide, crospovidone, microcrystalline cellulose, sodium bicarbonate, povidone K29 / 32 and part of metformin hydrochloride (50.0 mg); add poloxa with a concentration of 12.5% Poloxamer solution (prepared by dissolving 0.5mg of poloxamer 188 in 3.5g of purified water), granulated, dried, and pulverized to obtain premixed powder I, wherein the drying temperature should not be higher than 60°C, and the moisture content of premixed powder I Content ≤ 5wt%.
[0052] Step 2: Mix the premixed powder Ⅰ with mannitol, crospovidone, povidone K29 / 32, and the remaining metformin hydrochloride (450.0 mg), add a wetting agent, purified water, granulate, and dry to obtain dry granules A, the moisture content of the dried particles A is ≤3wt%.
[00...
Embodiment 2
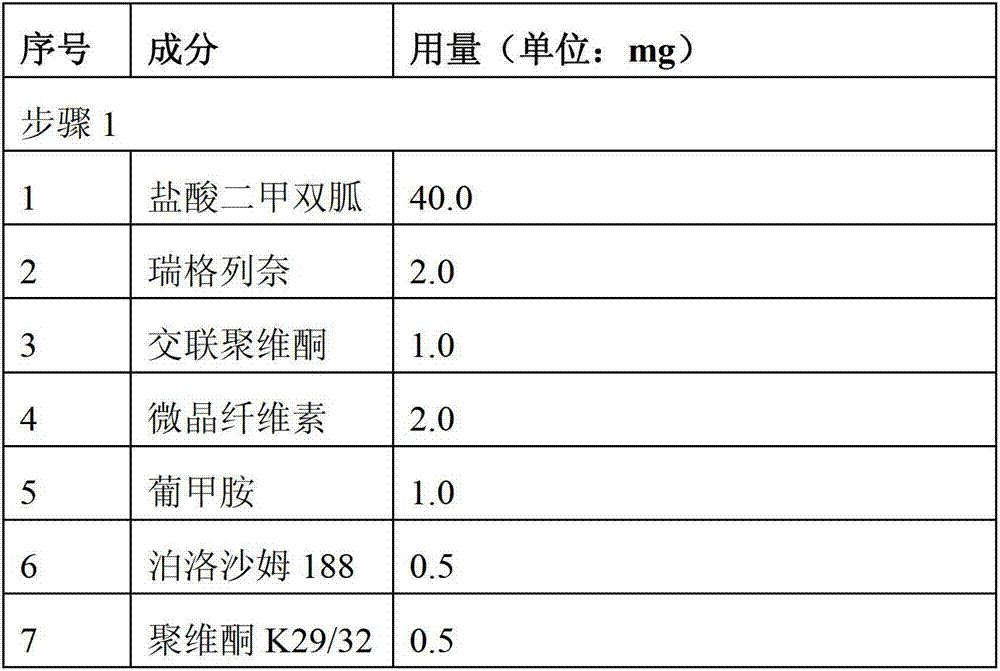
[0057] Preparation of single-dose pharmaceutical composition of repaglinide 2.0 mg and metformin hydrochloride 500 mg
[0058] Prepare according to the ingredients and dosage of each step in the table below:
[0059]
[0060]
[0061] Preparation method 1:
[0062] Step 1, mix repaglinide and meglumine evenly, then mix evenly with microcrystalline cellulose, crospovidone, povidone K29 / 32 and part of metformin hydrochloride (40.0 mg); use concentration of 8.9% The poloxamer solution (prepared by adding 0.5g of poloxamer 188 to 5.1g of 50% ethanol solution) was granulated, dried at 60°C until the moisture content was ≤3wt%, and pulverized to obtain premixed powder I.
[0063] Step 2. Mix the premixed powder I with the remaining metformin hydrochloride (460.0 mg), mannitol, crospovidone, and povidone K29 / 32 evenly, add purified water, granulate, dry, and granulate to obtain dry granules A (moisture content ≤ 3wt%).
[0064] Step 3. Add microcrystalline cellulose, crospov...
Embodiment 3
[0072] Preparation of single-dose pharmaceutical composition of repaglinide 2.0 mg and metformin hydrochloride 500 mg
[0073] Prepare according to the ingredients and dosage of each step in the table below:
[0074]
[0075]
[0076] Preparation:
[0077] Step 1, mix repaglinide and sodium bicarbonate evenly, then mix evenly with crospovidone, microcrystalline cellulose, povidone K25 and part of metformin hydrochloride (50.0mg); Dialkyl sodium sulfate solution (prepared by adding 0.5 mg sodium lauryl sulfate to 5.0 mg purified water) was granulated, dried at 60°C until the moisture content was ≤3%, and pulverized to obtain premix powder I.
[0078] Step 2. Mix the premixed powder I with mannitol, povidone K25, remaining metformin hydrochloride (450.0 mg) and cross-linked povidone evenly, add purified water, granulate, and obtain wet granules; take out the wet granules and dry until The moisture content of the granule is 1wt%-4wt%, and the dry granule A is obtained.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com