Antiallergic pharmaceutical composition and preparation method thereof
A composition and anti-allergic technology, which can be used in drug combinations, pharmaceutical formulations, allergic diseases, etc., and can solve the problems of cumbersome parameter setting, high preparation cost, and complex process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
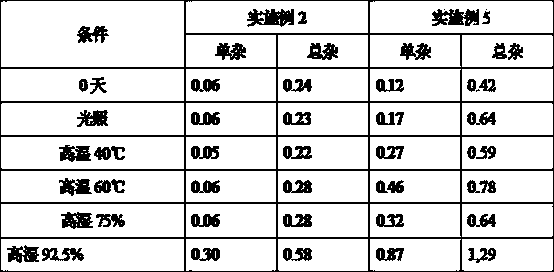
Image
Examples
Embodiment 1
[0018] Name of raw material Proportion(%) Levocetirizine Hydrochloride 5.0 lactose 63.5 silica 2.0 microcrystalline cellulose 27.5 Sucralose 1.0 essence 0.2 Magnesium stearate 0.8
[0019] Preparation Process:
[0020] 1. Mix the active ingredient levocetirizine hydrochloride and silicon dioxide, mix well, add microcrystalline cellulose and mix for about 5 minutes.
[0021] 2. The above mixture is subjected to dry granulation to obtain dry granules.
[0022] 3. Prepare 40% ethanol solution for later use.
[0023] 4. Mix the prescription amount of mannitol and other wet granulation auxiliary materials evenly, and use the pre-prepared wetting agent for wet granulation.
[0024] 5. Dry at 40-50°C, sieve through a 24-mesh sieve, calculate the yield, add sucralose, essence, and magnesium stearate, and mix well.
[0025] 6. Adjust the weight and pressure of the tablet, and press it into a tablet.
Embodiment 2
[0027] Name of raw material Proportion(%) Levocetirizine Hydrochloride 10.2 silica 6.0 lactose 63.5 microcrystalline cellulose 77.8 Sucralose 1.0 Menthol 0.2 Magnesium stearate 1.0
[0028] Preparation process: same as above.
Embodiment 3
[0030] Name of raw material Proportion(%) Levocetirizine Hydrochloride 5.0 lactose 39.0 microcrystalline cellulose 27.0 Mannitol 8.0 Low-substituted hydroxypropyl cellulose 17.0 Sucralose 1.0 Menthol 0.2 silica 2.0 Magnesium stearate 0.8
[0031] Preparation process: Same as above, both microcrystalline cellulose and low-substituted hydroxypropyl cellulose need to be added internally and externally.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com