CaCO3 vitamin D3 preparation and preparation method thereof
A vitamin and calcium carbonate technology, applied in pill delivery, pharmaceutical formulations, active ingredients of aluminum/calcium/magnesium, etc., can solve the problem of difficult mixing of vitamin D3, sensitivity to light, heat, and oxygen, and unqualified content uniformity of vitamin D3 And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Based on this, an embodiment of the present invention provides a calcium carbonate vitamin D3 preparation and a preparation method thereof. The preparation method of the calcium carbonate vitamin D3 preparation comprises the following steps S1-S5.
[0036] Step S1: providing raw materials, including calcium carbonate, vitamin D3, disintegrants, fillers, binders, lubricants and coating powders.
[0037] In one embodiment, the raw materials include 100 parts by weight of calcium carbonate, 0.0002-0.0005 parts by weight of vitamin D3, 4-10 parts by weight of disintegrants, 30-50 parts by weight of fillers, 10-20 parts by weight of binder, 2-5 parts by weight of lubricant and 4-10 parts by weight of coating powder.
[0038] Further, the disintegrant is at least one of sodium carboxymethyl starch, crospovidone and croscarmellose sodium, preferably sodium carboxymethyl starch. The filler is at least one of sorbitol and mannitol, preferably sorbitol. The binder is at least ...
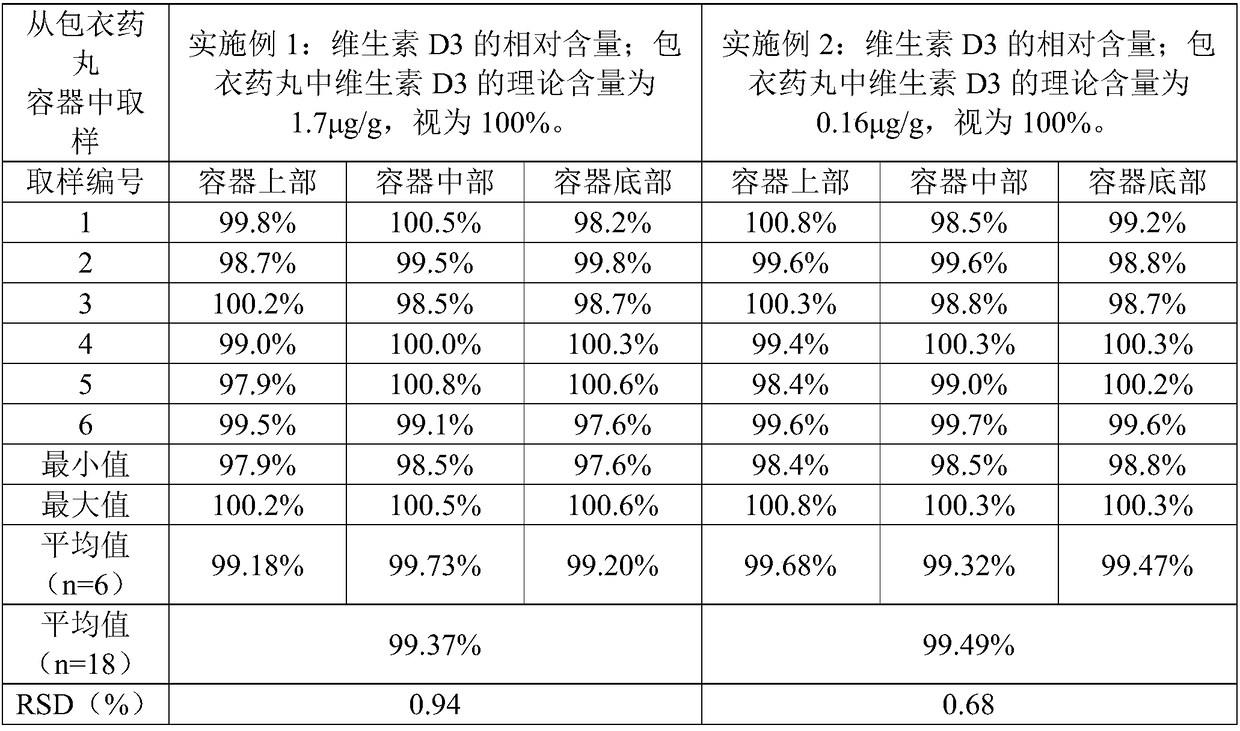
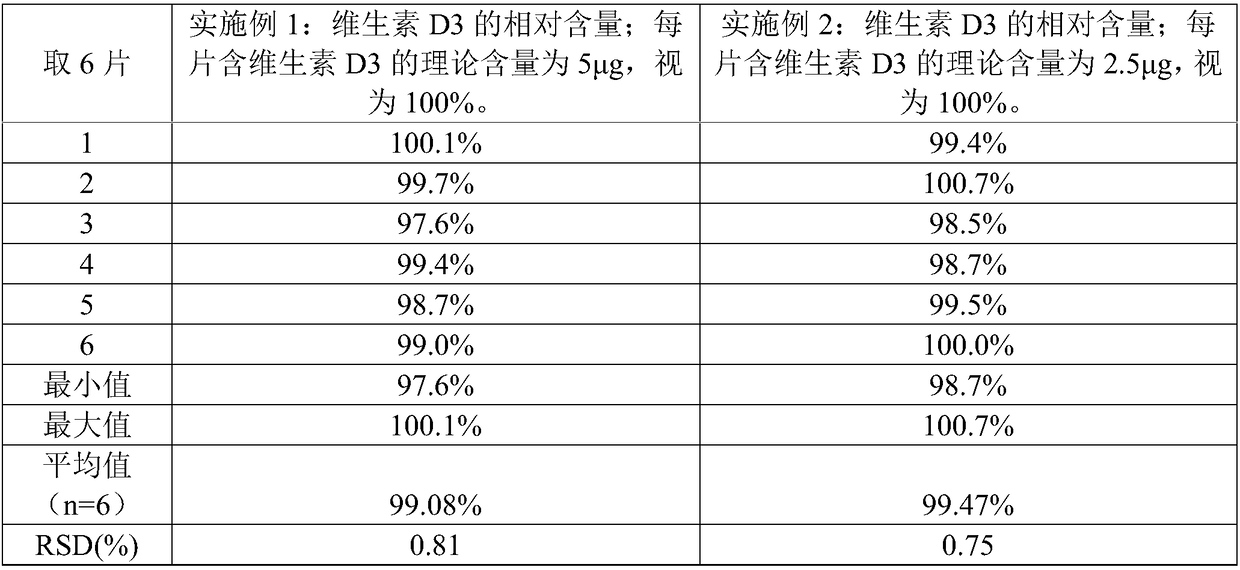
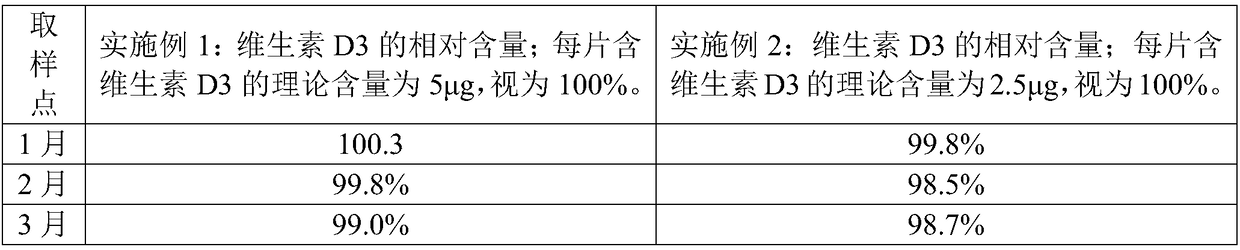
Embodiment 1
[0065] A preparation method of calcium carbonate vitamin D3 chewable tablet, comprising the steps of:
[0066] 1) raw material is provided, and this raw material comprises the calcium carbonate of 100 weight parts, the vitamin D3 of 0.0003 weight part, the sodium starch glycolate of 6 weight parts, the sorbitol of 40 weight parts, the povidone K30 of 15 weight parts, the povidone K30 of 3 weight parts The magnesium stearate of 8 parts by weight, the Opadry Y-1-7000 of 8 parts by weight, the essence of 0.2 parts by weight and the aspartame of 0.1 parts by weight.
[0067] 2) Sieve the above-mentioned calcium carbonate, sodium carboxymethyl starch and sorbitol, and mix evenly to obtain a mixture. The above-mentioned povidone K30 is equally divided into two parts according to the mass equivalent, and a part of povidone K30 is dissolved in 75% (v / v) ethanol aqueous solution to prepare 75% (w / w) povidone K30 containing 15% (w / w) povidone K30 % (v / v) ethanol in water, as a binder s...
Embodiment 2
[0078] Embodiment 2 is substantially the same as Example 1, and the difference is that the raw materials of step 1) include the calcium carbonate of 100 parts by weight, the vitamin D3 of 0.0004 parts by weight, the sodium carboxymethyl starch of 10 parts by weight, the sorbic acid of 50 parts by weight Alcohol, the Povidone K30 of 10 parts by weight, the magnesium stearate of 5 parts by weight, the Opadry Y-1-7000 of 4 parts by weight, the essence of 0.1 parts by weight and the Aspartame of 0.2 parts by weight;
[0079] Step 2) Divide the above-mentioned povidone K30 into three equal parts according to the mass, take two parts in step 2), and take the remaining part in step 4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com