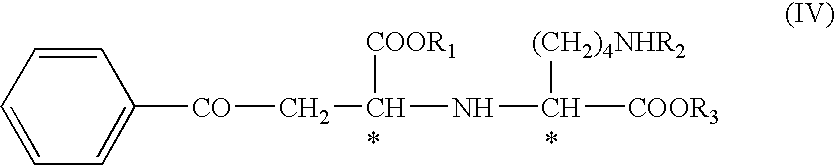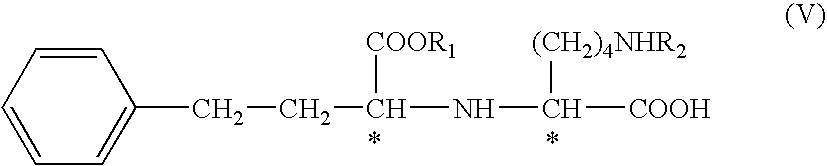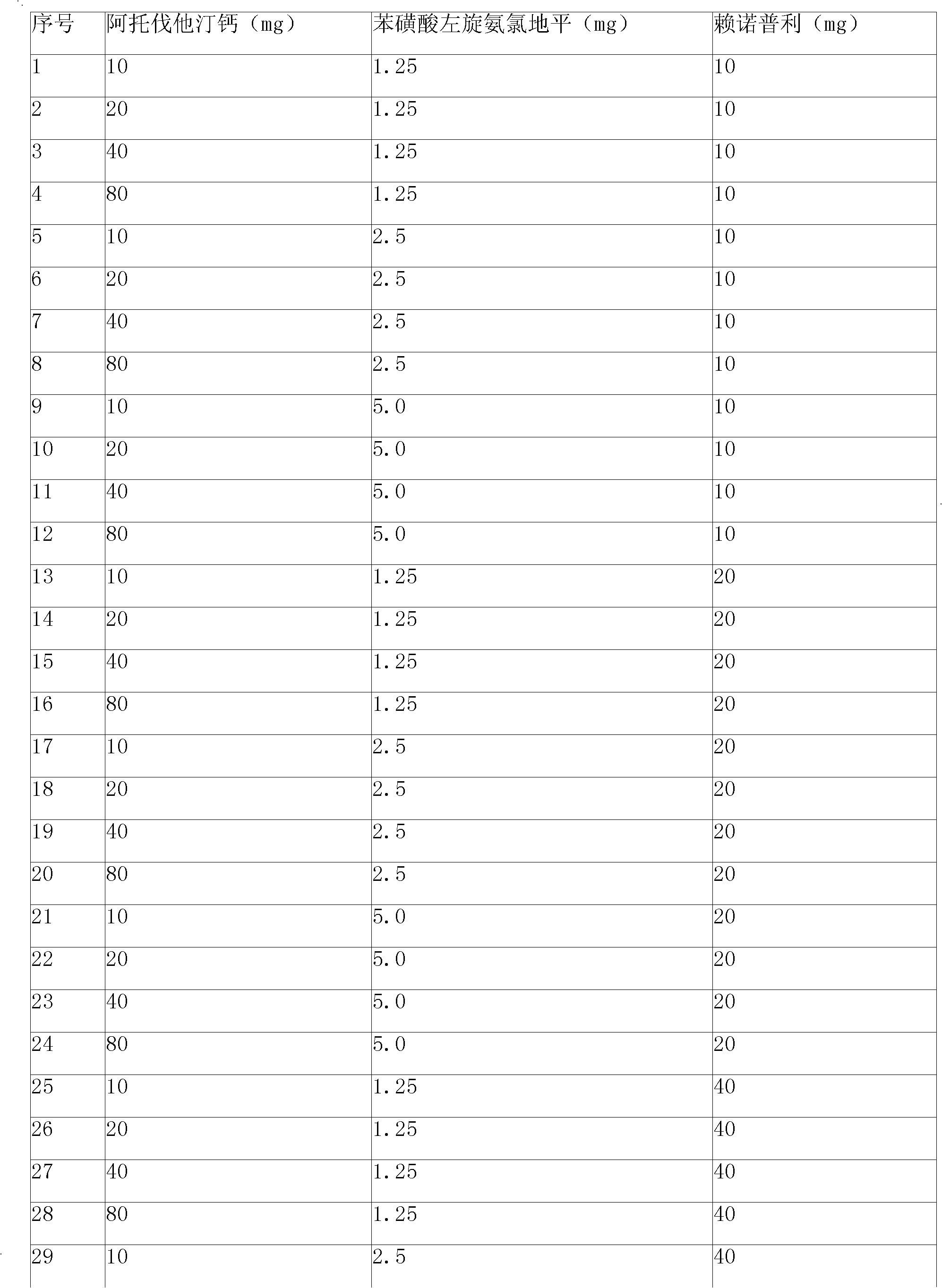Patents
Literature
64 results about "Lisinopril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
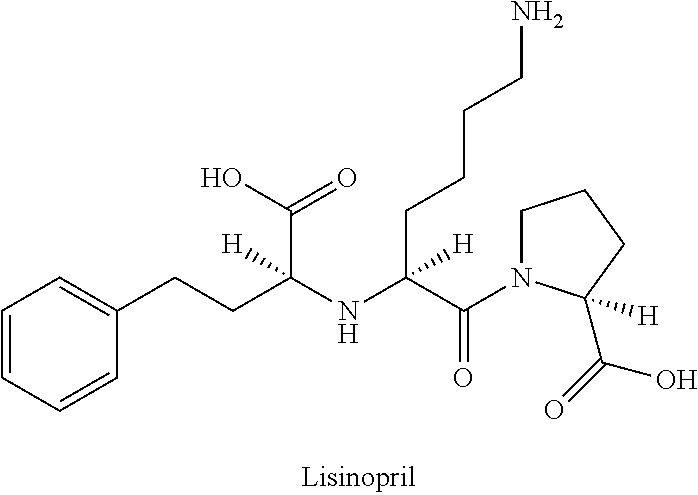
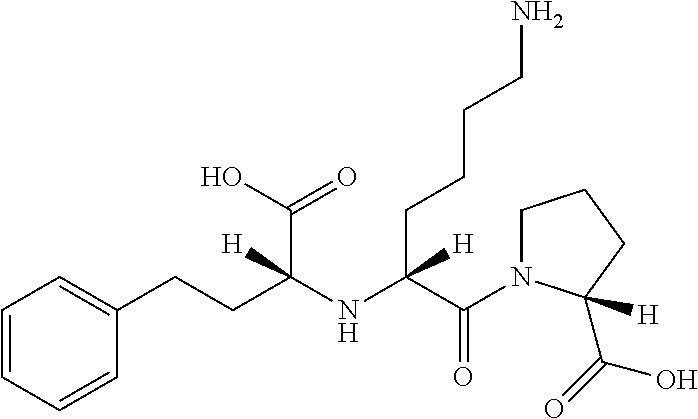
Lisinopril is used to treat high blood pressure.
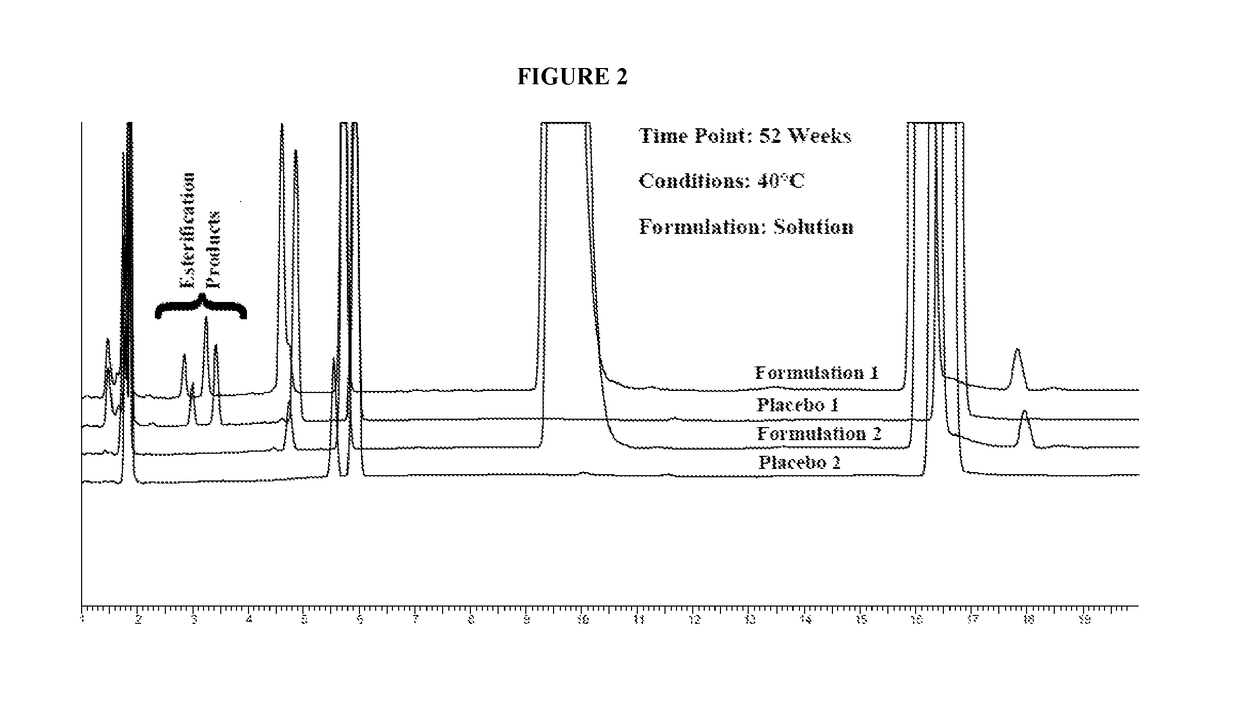
Stable pharmaceutical compositions containing an ACE inhibitor
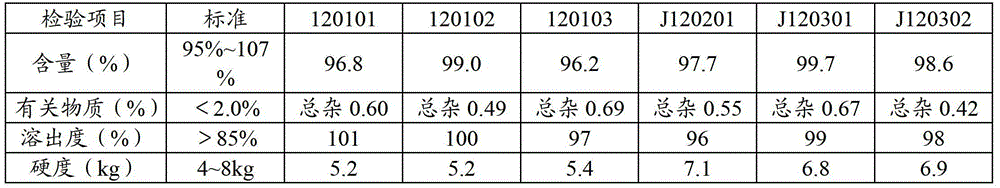
InactiveUS6869963B2Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalInstability
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Lisinopril formulations
Provided herein are stable lisinopril oral liquid formulations. Also provided herein are methods of using lisinopril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and acute myocardial infarction.
Owner:SILVERGATE PHARMA
Immobilized whole-cell catalyst and its preparation method and application
InactiveCN102260664ALow costEasy to operateBacteriaMicroorganism based processesPtru catalystLisinopril
The invention relates to the field of biotechnology in the pharmaceutical industry, and discloses the preparation of an immobilized whole-cell catalyst and its application in the synthesis of a Puley intermediate (R)-2-hydroxy-4-phenyl-butyric acid. The recombinant cells containing the D-type lactate dehydrogenase gene and the formate dehydrogenase gene are established and fixed to prepare the immobilized whole cell catalyst. Using this catalyst to synthesize the intermediate (R)-2-hydroxy-4-phenyl-butyric acid of lisinopril, the reaction conditions are mild, the action is specific, and there are no by-products. It is used in medicine and food industries. value.
Owner:SYNCOZYMES SHANGHAI
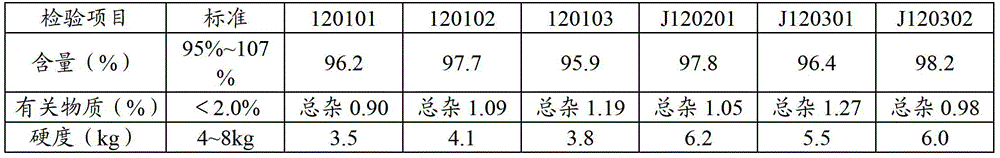
Stable pharmaceutical compositions containing an ace inhibitor
InactiveUS20050009806A1Extended shelf lifeMinimize impactBiocidePill deliveryAlkaline earth metalDepressant
A stable pharmaceutical composition comprising about 1 wt. % to about 80 wt. % of an ACE inhibitor or a pharmaceutical acceptable salt thereof, about 1 wt. % to about 70 wt. % of an alkali or alkaline earth metal carbonate, and about 1 wt. % to about 80 wt. % of hydroxypropyl cellulose, wherein the ACE inhibitor is selected from the group consisting of quinapril, enalapril, spirapril, ramipril, perindopril, indolapril, lisinopril, alacepril, trandolapril, benazapril, libenzapril, delapril, cilazapril and combinations thereof; wherein the formation of an internal cyclization product, and / or ester hydrolysis product, and / or oxidation product, has been reduced or eliminated, and the weight percents are based on the total weight of the pharmaceutical composition. The stabilized pharmaceutical compositions of the invention exhibit a number of advantages as follows: (i) the ACE inhibitor or a pharmaceutical acceptable salt thereof present in the compositions is preserved from degradation; (ii) the compositions exhibit extended shelf-life under normal storage conditions; (iii) the effect of moisture on the compositions is minimized; (iv) the compositions exhibit minimal, if any, discoloration over a significant period of time; and (v) the compositions exhibit minimal, if any, instability when employed in the presence of colorants.
Owner:SANDOZ AG
Lisinopril formulations
Provided herein are stable lisinopril oral liquid formulations. Also provided herein are methods of using lisinopril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and acute myocardial infarction.
Owner:AZURITY PHARMA INC
Method for preparing lisinopril intermediate
InactiveCN102617704AAvoid it happening againIncrease contentOrganic compound preparationCarboxylic acid amides preparationSolventLisinopril
The invention relates to a method for preparing a lisinopril intermediate. The method comprises the following steps of: mixing and stirring N-protecting group-L-lysine or N-protecting group-L-lysine-L-proline, sodium triacetoxyborohydride, glacial acetic acid and a reaction solvent uniformly; cooling, dripping a mixed solution of alpha-oxo-phenylbutyrate and the reaction solvent, and continuing to react; reacting at room temperature for 2 to 6 hours; and adding water, extracting, recrystallizing, filtering and drying to obtain the lisinopril intermediate, wherein the sequence of dripping raw materials and the sequence of reaction can be adjusted. According to the method, NaBH3CN is replaced by NaBH(OAc), so that the generation of toxic side products is avoided; and the method is environment-friendly, and the reaction is performed under normal pressure, so that the efficiency and safety are improved, the content of an S isomer in the obtained product is improved, and the cost is reduced.
Owner:JIANGXI DIRUI SYNTHETIC CHEM
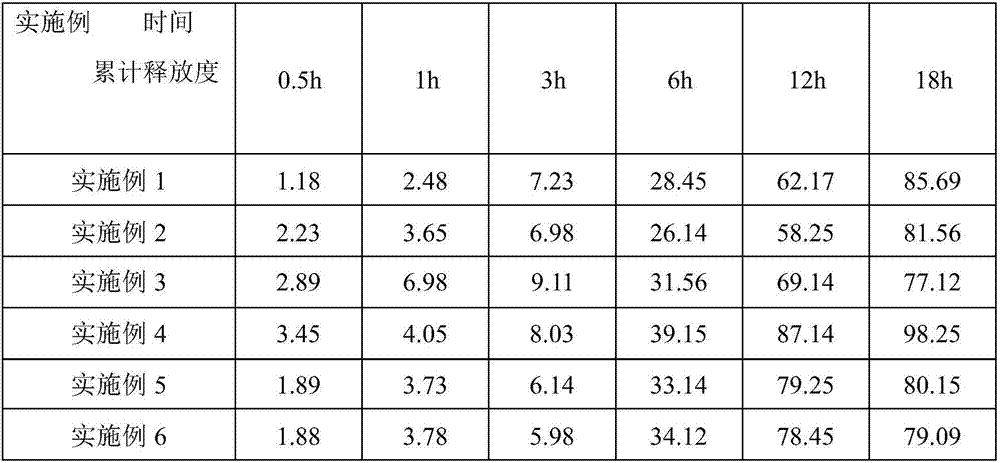
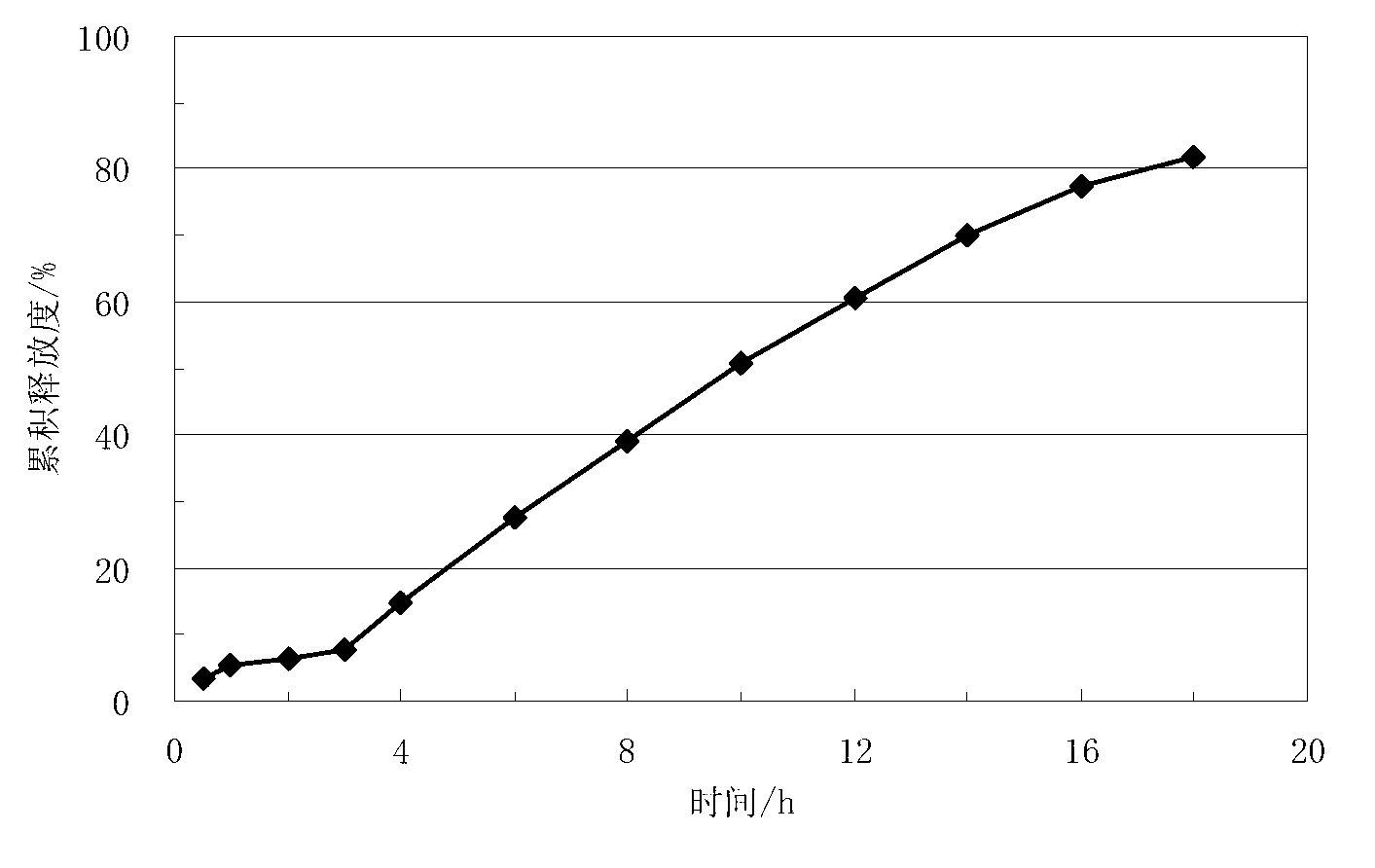
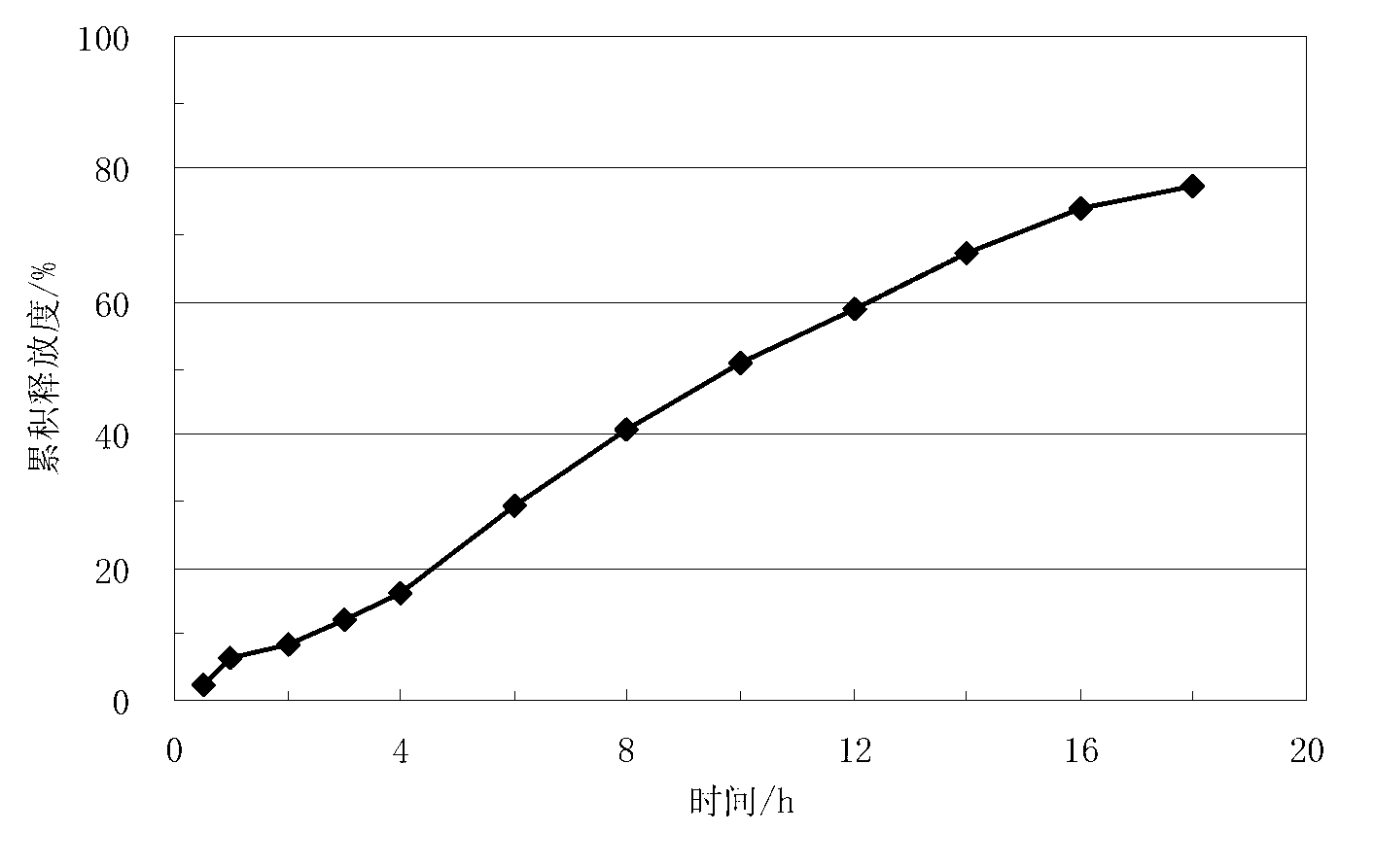
Lisinopril controlled-release tablet and preparation method thereof
InactiveCN103006612AThe effect of delayed releaseAchieve the effect of releaseDipeptide ingredientsPharmaceutical delivery mechanismFoaming agentWater insoluble
The invention relates to a lisinopril controlled-release tablet and a preparation method thereof, belonging to the field of pharmaceutic preparation technology and solving the problem that existing lisinpopril controlled-release tablet cannot delay release and release mildly. The tablet consists of core of the tablet containing lisinopril and a coating wrapped outside the core of the tablet, the core of the tablet containing the lisinopril comprises the following components by weight percent: 5.0%-20% of lisinopril, 25%-55% of framework material, 20%-50% of filler, and1.0%-3.0% of lubricant, the coating comprises the following components in weight percent: 60%-90% of water insoluble coating material and 10%-40% of pore-foaming agent. The method comprises the preparation of core of the tablet containing lisinpopril, the preparation of coating liquor, and the preparation of the tablet by wrapping the coating liquor outside the core of table so as to form the coating. The lisinopril controlled-release tablet can delay release and release mildly, and the method is simple and beneficial to industrialization.
Owner:石雷 +1
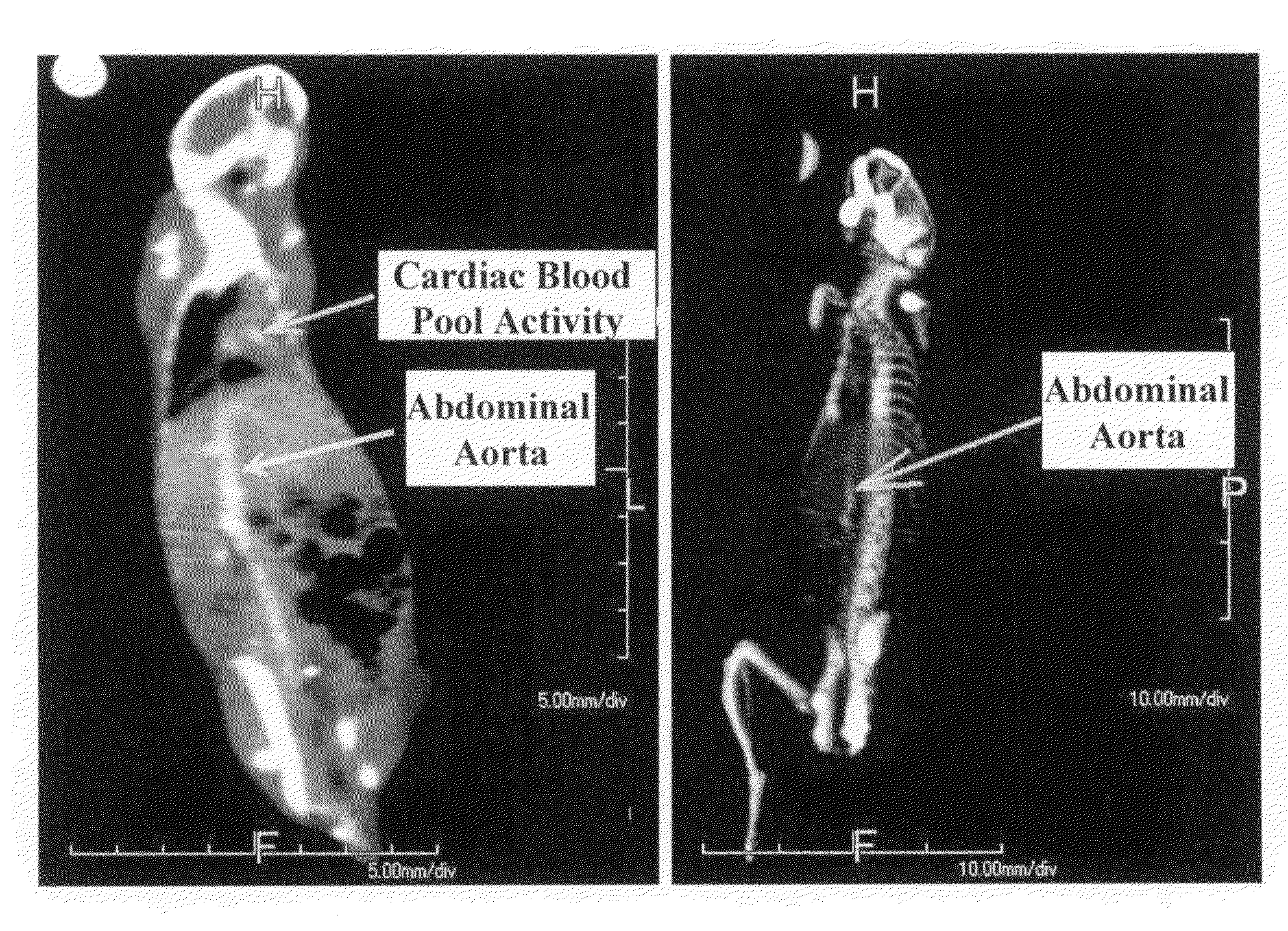
Gold nanoparticle imaging agents and uses thereof
Overexpression of angiotensin-converting enzyme (ACE) has been associated with a number of pathophysiologies, including those associated with cancer and the cardiovascular system. Thus, targeted imaging of ACE is of crucial importance for monitoring tissue ACE activity as well as treatment efficacy. To this end, lisinopril-coated gold nanoparticles were prepared to provide a new type of probe for targeted molecular imaging of ACE by tuned K-edge computed tomography (CT) imaging.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
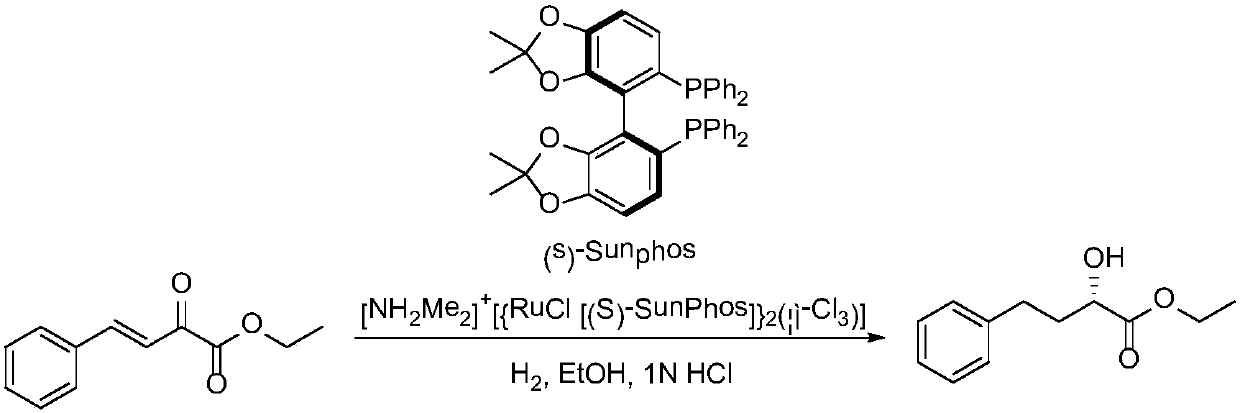
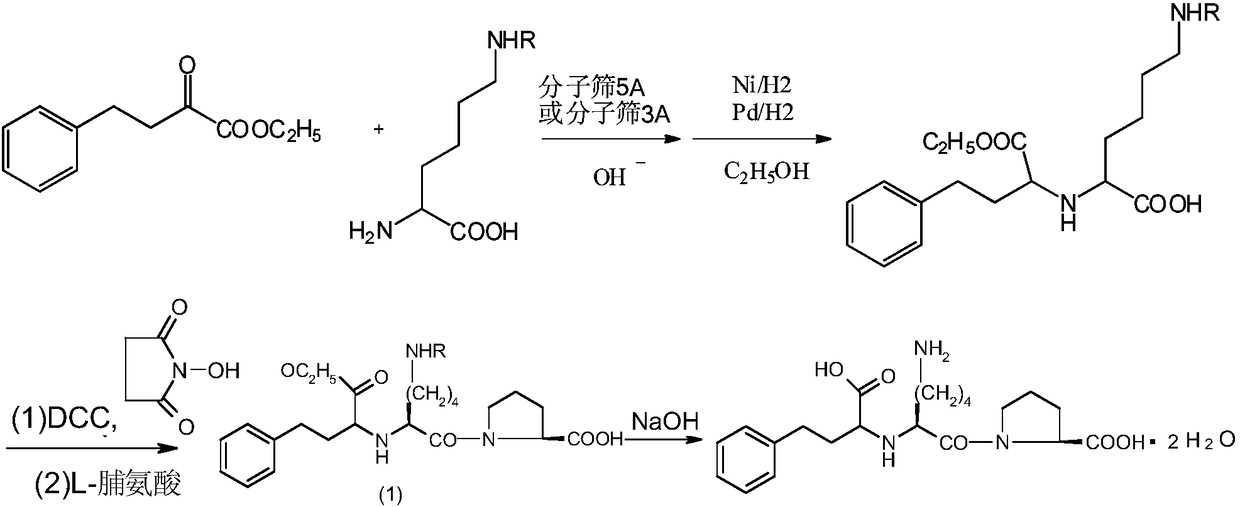
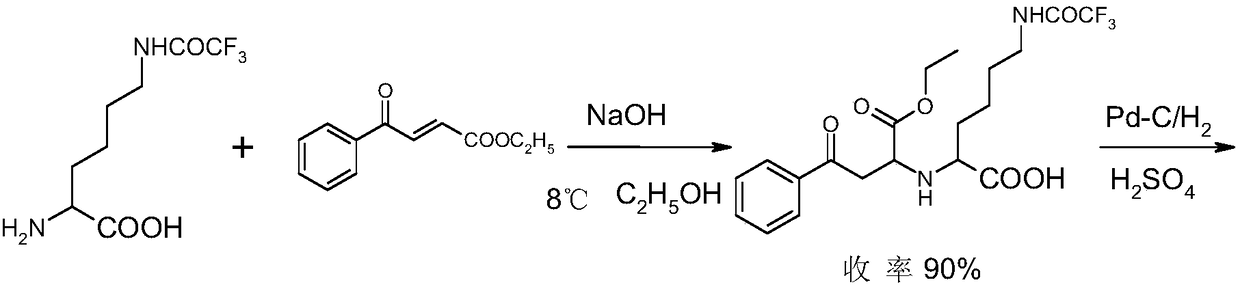
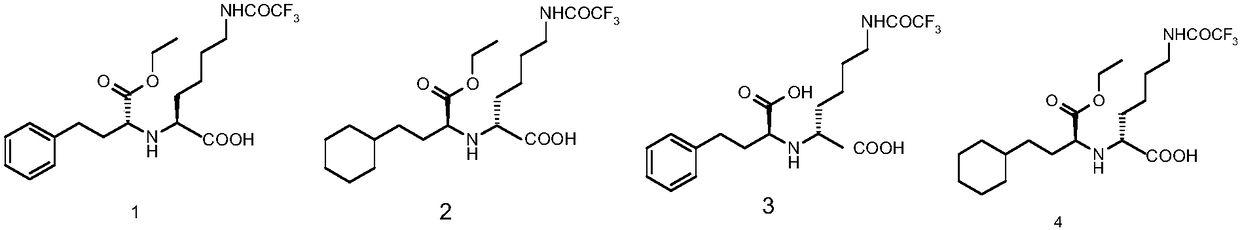
Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride
ActiveCN1611494AReduce dosageReduce manufacturing costOrganic chemistryChemical synthesisOrganic solvent
The invention relates to a kind of chemosynthesis method of N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine-N-hydroxy acid anhydride, which is the medicine midbody for synthesizing Pulitzer series medicine such as Enalapril, Ramipril, Lisinopril and so on. The invention uses couple (trichloromethyl) ester carbonate and N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine as row material, and get the product by reacting with catalyst action in organic solvent. This chemosynthesis method is a manufacturing method of N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine-N-hydroxy acid anhydride, which has acquirable raw material, low production cost, and no three wastes normally.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of N-carboxyalkyl dipeptide type angiotensin converting enzyme inhibitor
InactiveCN1429835ASimple and efficient processSuitable for industrialized mass productionDipeptidesCardiovascular disorderDipeptideDepressant
A process for preparing N-carboxyalkyl dipeptide type angiotensin converting enzyme (ACE) depressant (such as enalapril maleate, ramipril, etc) includes such steps as preparing N-carboxylic acid anhydride from bis (trichloromethyl) carbonate, and coupling with relative alpha-amino acid.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Compound blood pressure reducing prepn containing angiotonin converzyme inhibitor, calcium ion agonist and Estazolam
InactiveCN1526398AGood curative effectLittle side effectsOrganic active ingredientsPill deliveryCaptoprilSide effect
The present invention provides one new kind of compound blood pressure reducing preparation containing angiotonin converzyme inhibitor, calcium ion agonist, Estazolam and pharmaceutically acceptable carrier. The angiotonin converzyme inhibitor is selected from Enalapril, Ramipril, Benalapril, Lisinopril, Acertil, etc. as well as their mixture; and the calcium ion agonist is selected from Nitrendpine, Amlodipine Besylate, Nifedipine, Felodipine, etc. as well as their mixture. The present invention utilizes the synergistic effect between different medicines to raise the blood pressure lowering effect, reduce side effect and improve the compliance of patient.
Owner:杜晓锋
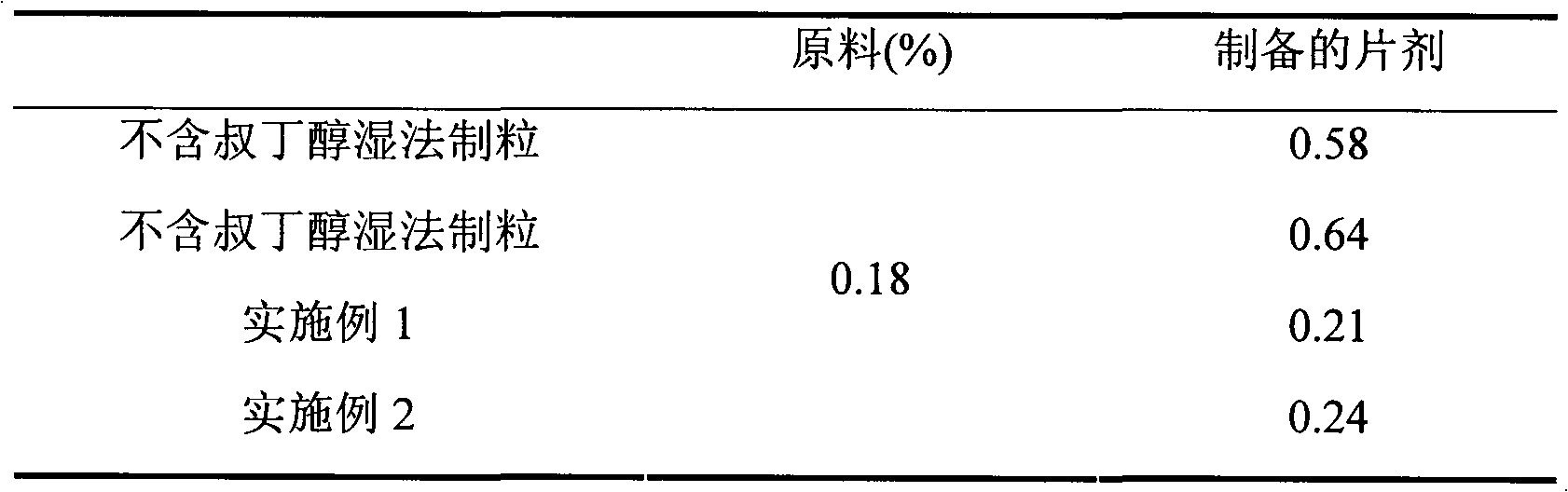
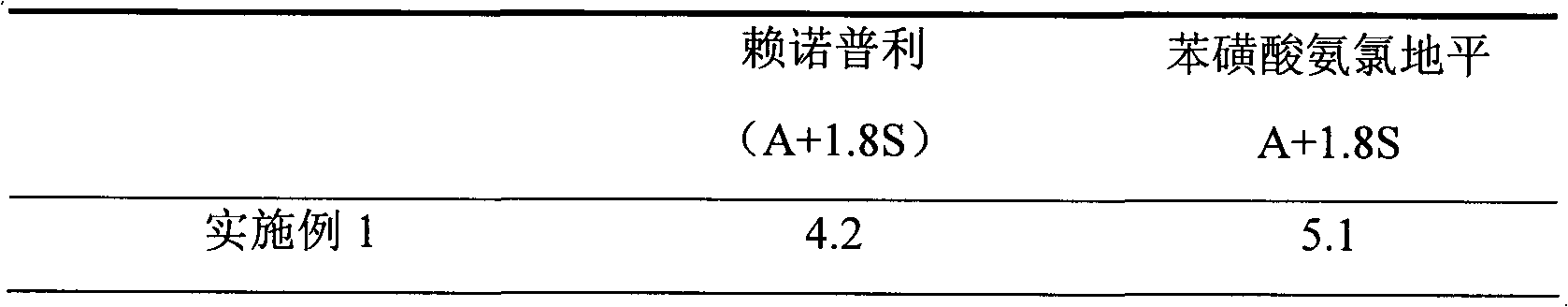
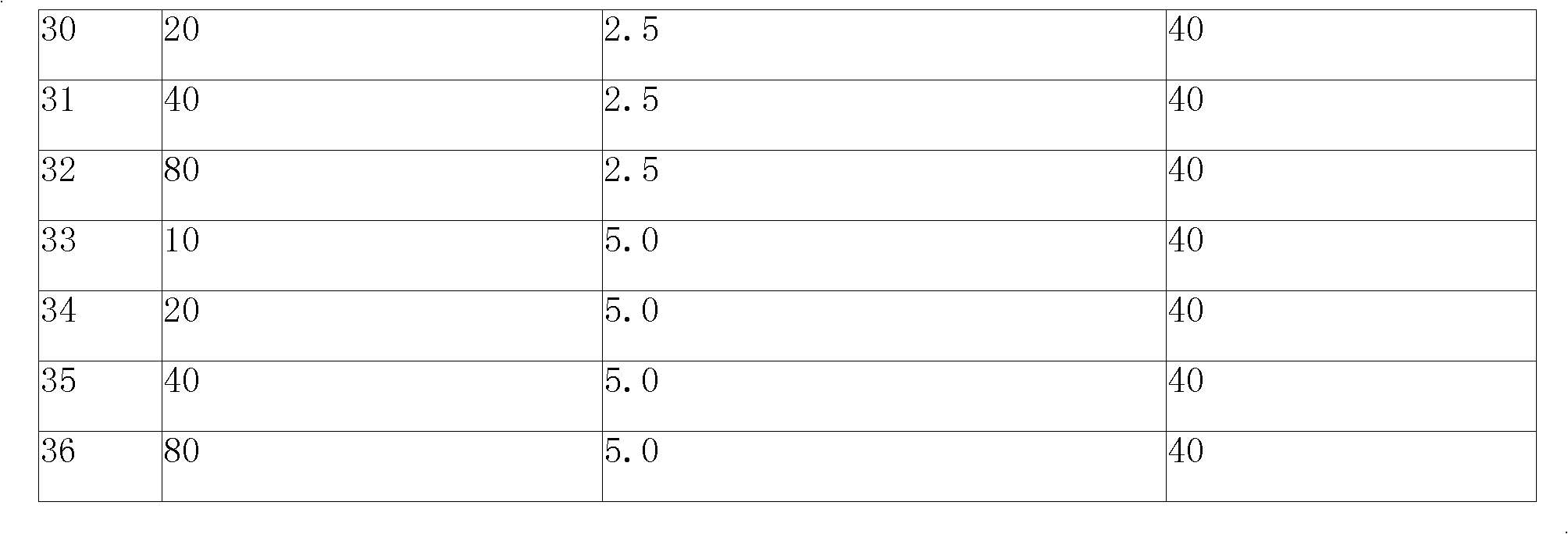
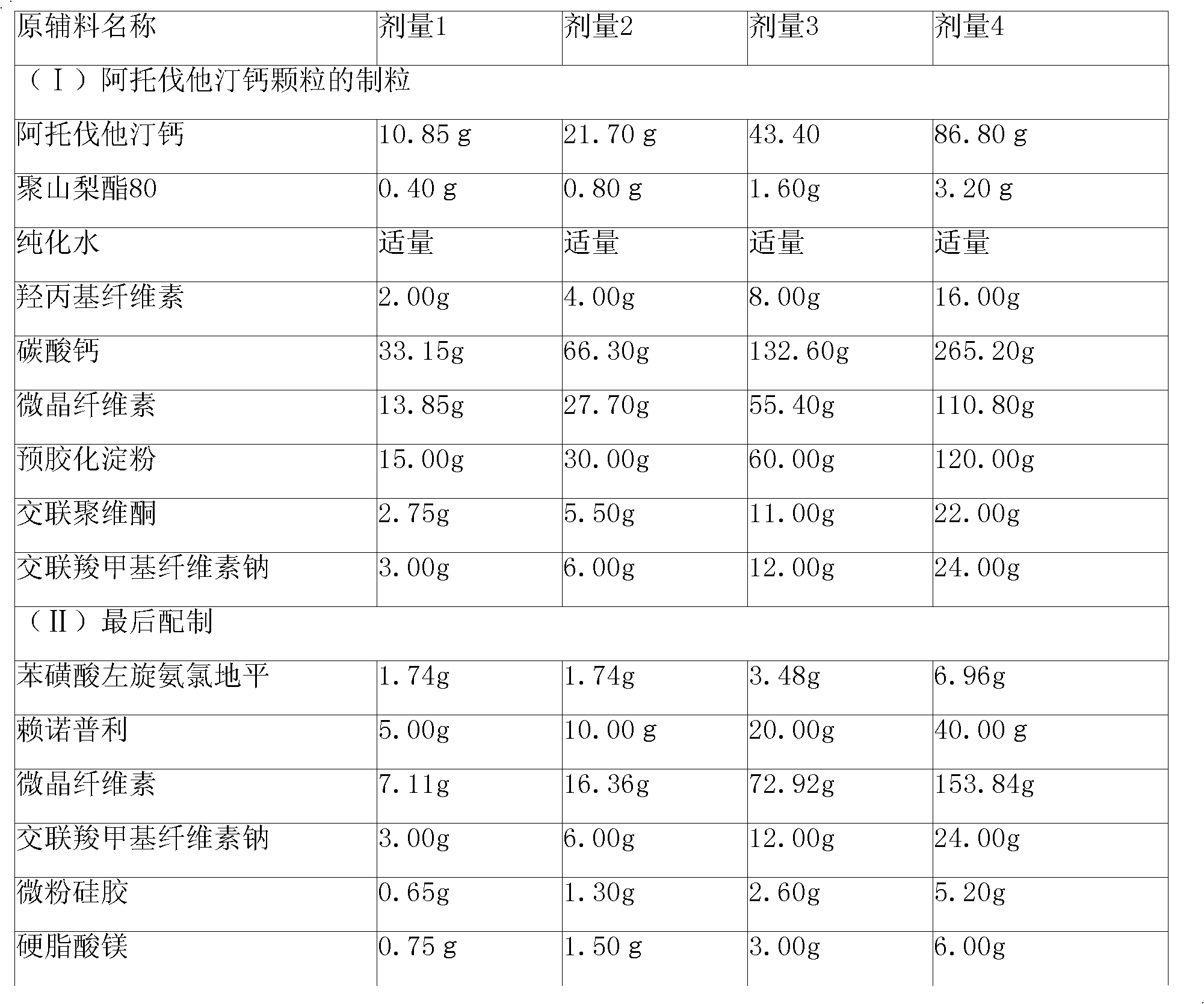
Preparation method of compound amlodipine-lisinopril tablets
ActiveCN102423482ASolve the problems of poor content uniformity and high energy consumption of dust in direct compressionAddress issues affecting lisinopril stabilityOrganic active ingredientsDipeptide ingredientsAdhesiveActive component
The invention discloses a prescription and a preparation technology of compound tablets containing amlodipine besylate and lisinopril. According to the invention, amlodipine besylate and lisinopril are adopted as active components, a tert-butyl alcohol solution is adopted as an adhesive, and the tablets are prepared through wet granulating and tabletting. The content uniformity of the compound tablets is relatively good. Lisinopril is stable in the preparation process, and the content of the related substances of lisinopril is low.
Owner:南京海鲸药业股份有限公司
Lisinopril-containing compound preparation for treating hypertension
ActiveCN102008710AQuick resultsHigh blood pressureOrganic active ingredientsDipeptide ingredientsSide effectLevamlodipine
The invention relates to a lisinopril-containing compound preparation for treating hypertension, which comprises the main components of L-amlodipine or pharmaceutically acceptable salt thereof, hydrochlorothiazide and lisinopril or pharmaceutically acceptable salt thereof, and the auxiliary component of a pharmaceutically acceptable carrier, wherein the content of the L-amlodipine or the pharmaceutically acceptable salt thereof in unit preparation is 2.5-10.0mg. The invention has the advantages of fully exerting medicine compensation action mechanism through medicine combination therapy, increasing curative effect, quickly reaching the standards, making the compliance rate of blood pressure reach 82%, reducing adverse effect associated with the dose increase of certain medicine and keeping longer action time. The compound preparation has the characteristics of quick effect taking, high compliance rate of blood pressure, little side effect and low cost.
Owner:YUANHE PHARMA CO LTD
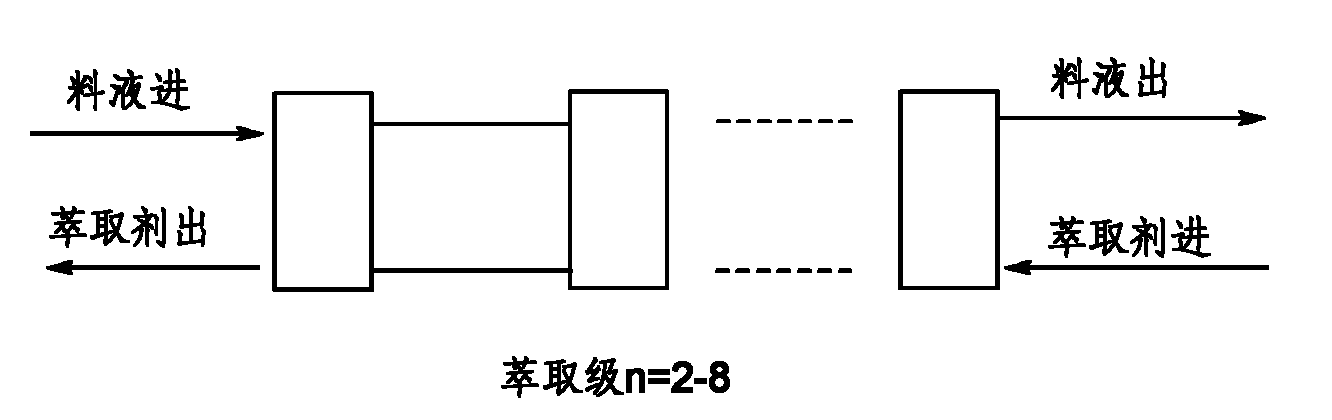
Method for separating lisinopril ester
InactiveCN101985462AEasy to separateEfficient recyclingPeptide preparation methodsOrganic solventEconomic benefits
The invention discloses a method for separating lisinopril ester. The method comprises the following steps of: performing annular gap type centrifugal extraction on lisinopril ester-containing material liquid by using a two to eight-stage serial countercurrent centrifugal extraction system consisting of annular gap types centrifugal extractors and by taking an organic solvent as an extracting agent; and concentrating the obtained extract phase and recovering the organic solvent to obtain the lisinopril ester. Compared with the conventional production process, the method has the advantages of a small amount of solvent, stable quality, high extraction rate, short period, low three-waste energy consumption (waste water, waste gas and industrial residues), safe and reliable production, and high implementation value and social economic benefit.
Owner:ZHEJIANG UNIV OF TECH +1
Lisinopril compound preparation for treating cardiovascular disease and preparation method thereof
InactiveCN107029208AQuick resultsHigh blood pressureOrganic active ingredientsDipeptide ingredientsSide effectLevamlodipine
The invention relates to a compound preparation for treating cardiovascular disease and in particular relates to a lisinopril compound preparation for treating cardiovascular disease. The lisinopril compound preparation comprises the following drugs in parts by weight: 2-6 parts of lisinopril, 0.8-2.8 parts of hydrochlorothiazide and 1.6-3.6 parts of levamlodipine besylate. According to an acting mechanism of giving full play to complementation of drugs by combined medication, the curative effect is increased, and the standard is quickly achieved, the rate of reaching the standard of blood pressure reaches 82%, and adverse effects related to increase of some dosage are reduced. The compound preparation has the characteristics of being quick to take effect, high in rate of reaching the standard of blood pressure, small in side effect and low in cost.
Owner:江苏黄河药业股份有限公司
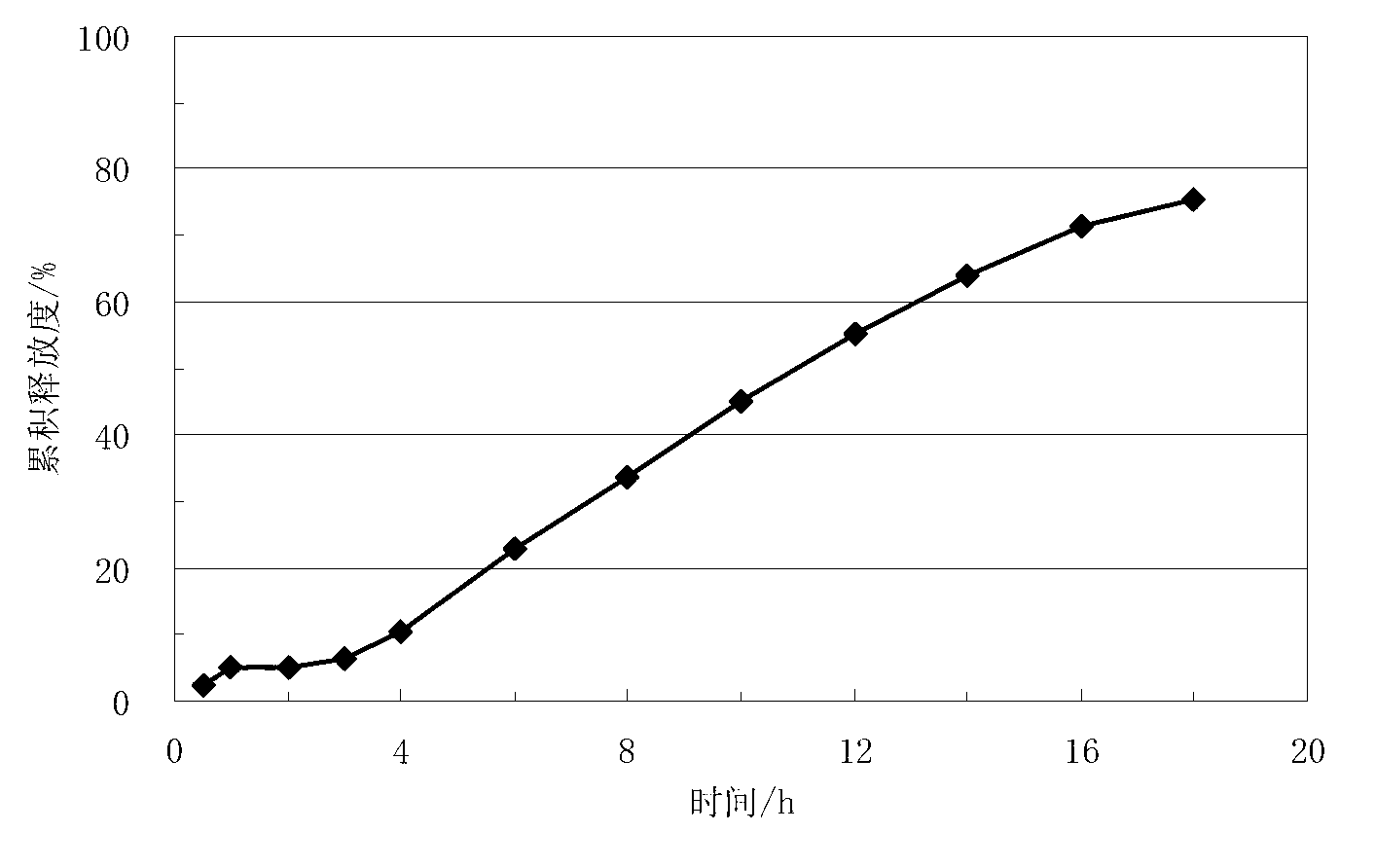
Lisinopril sustained release tablet and preparation method thereof
InactiveCN107281461AControl release speedFacilitated releaseDipeptide ingredientsPharmaceutical delivery mechanismSustained Release TabletPorous carbon
The invention relates to the technical fields of sustained release tablets and preparation thereof and particularly relates to a lisinopril sustained release tablet and a preparation method thereof. The lisinopril sustained release tablet is formed by a tablet core containing lisinopril and a coating layer packaging the tablet core, wherein the coating layer is prepared from non-water-soluble coating materials. The lisinopril sustained release tablet is characterized in that the tablet core contains the following substances in parts by weight: 5.0%-10 parts of lisinopril and 40-70 parts of a porous carbon material. The lisinopril sustained release tablet has a release delaying effect and a sustained release effect; and the preparation method is simple in process, wide in raw material source and beneficial to industrial production.
Owner:江苏黄河药业股份有限公司
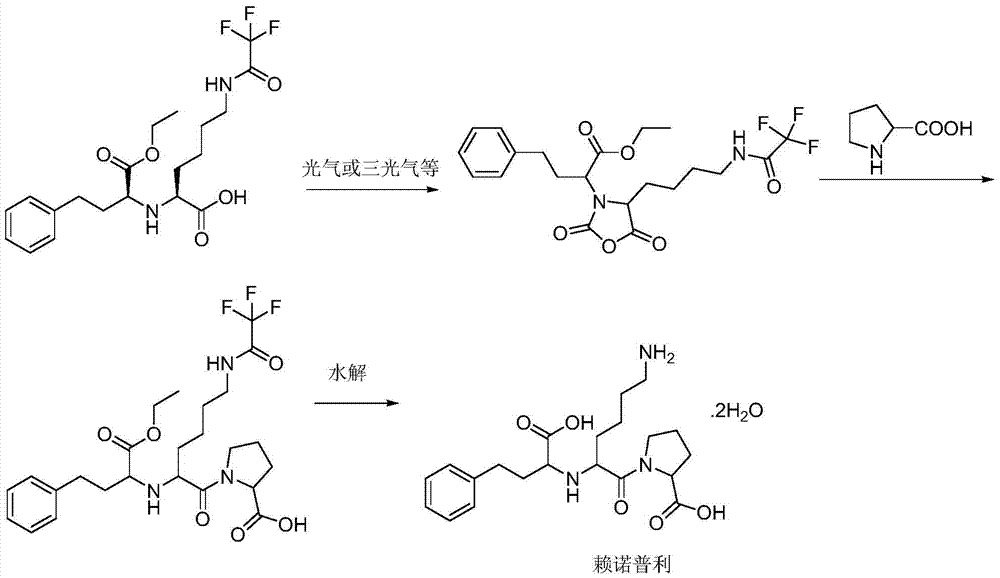
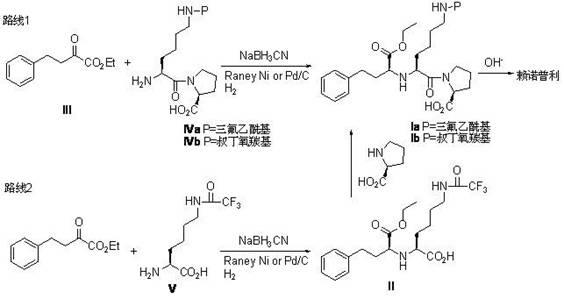
Process for the production of lisinopril
InactiveUS20070093664A1Organic compound preparationCarboxylic acid amides preparationSodium iodideLisinopril
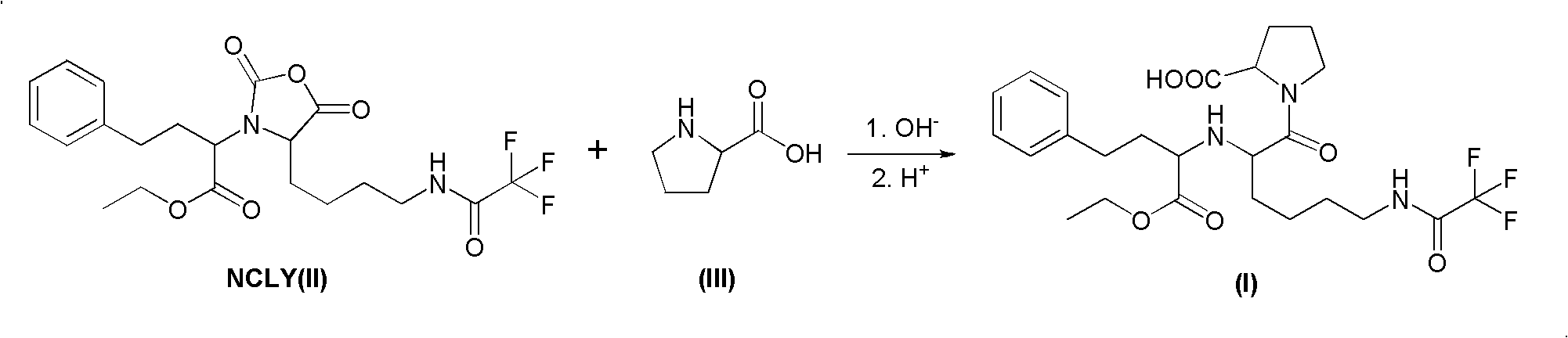
The present invention provides a process for preparing N2-[1(S)-ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysine and lisinopril thereof. Lisinopril shows excellent angiotensin converting enzyme inhibitor activity. Friedel-Crafts acylation of benzene with maleic anhydride in the presence of AlCl3 affords trans-β-benzoylacrylic acid. Treatment of benzoylacrylic acid with HCl gas in ethanol gives ethyl 2-chloro-4-oxo-4-phenylbutyrate in high yield. The coupling reaction between ethyl 2-chloro-4-oxo-4-phenylbutyrate and trifluoroacetyl-L-lysine benzyl ester in the presence of a base pair and sodium iodide produces alkyl lydine derivative with a good diastereoselectivity. Catalytic hydrogenation of lysine derivative with palladium gives N2-[1(S)-ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysine. This intermediate is activated to form cyclic N-anhydride by using N,N-carbonyldiimidazole and coupled with L-proline methyl ester hydrochloride to give fully protected lisinopril derivative, which is converted into crude lisinopril by hydrolysis.
Owner:ECZACIBASI ZENTIVA KIMYASAL URUNLER SANAYI VE TICARET
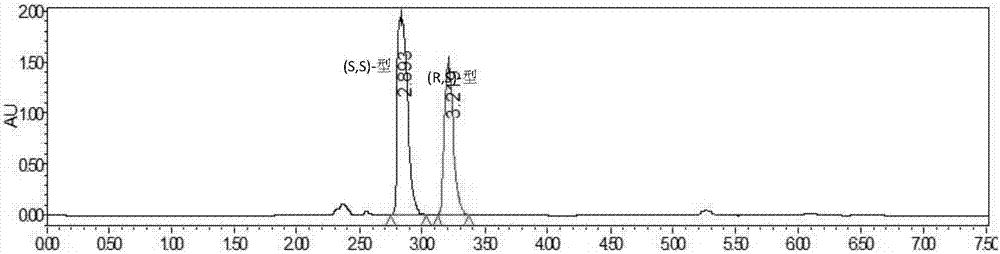
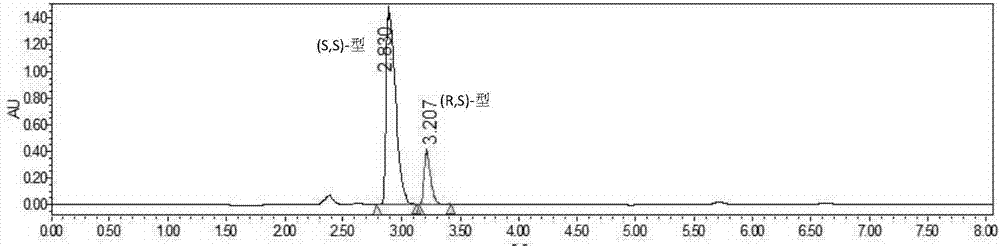
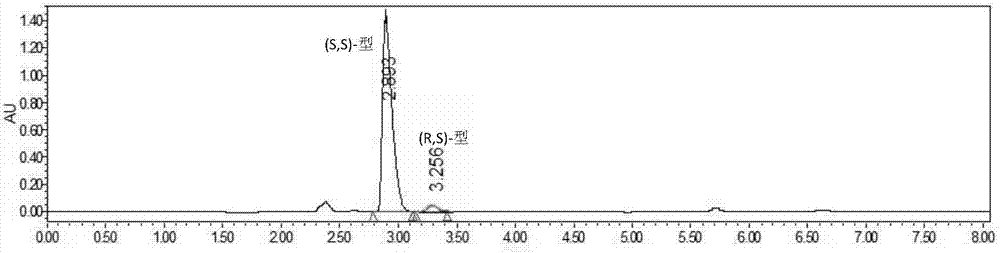
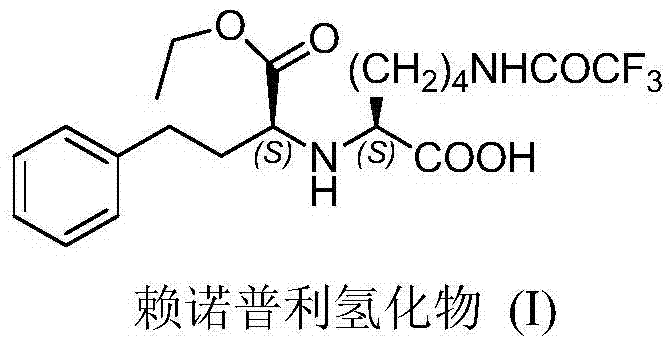
Method for resolving Lisinopril hydride through biocatalysis
The invention belongs to the technical field of biocatalysis, and relates to a method for resolving Lisinopril hydride through biocatalysis. The method for resolving Lisinopril hydride through biocatalysis comprises the following steps: by taking Lisinopril hydride as a substrate raw material, adding a proper volume of water, generating selective esterolysis (R,S)- Lisinopril hydride under the condition of existence of lipase, and retaining (S,S)- Lisinopril hydride; after the reaction is completed, separating a mixed solution to obtain the (S,S)- Lisinopril hydride. The method has the advantages of being high in catalysis efficiency, good in stereoselectivity, easy to separate enzyme from substrate, capable of recycling enzyme, low in cost, small in environment pollution, and the like.
Owner:CHANGXING PHARMA
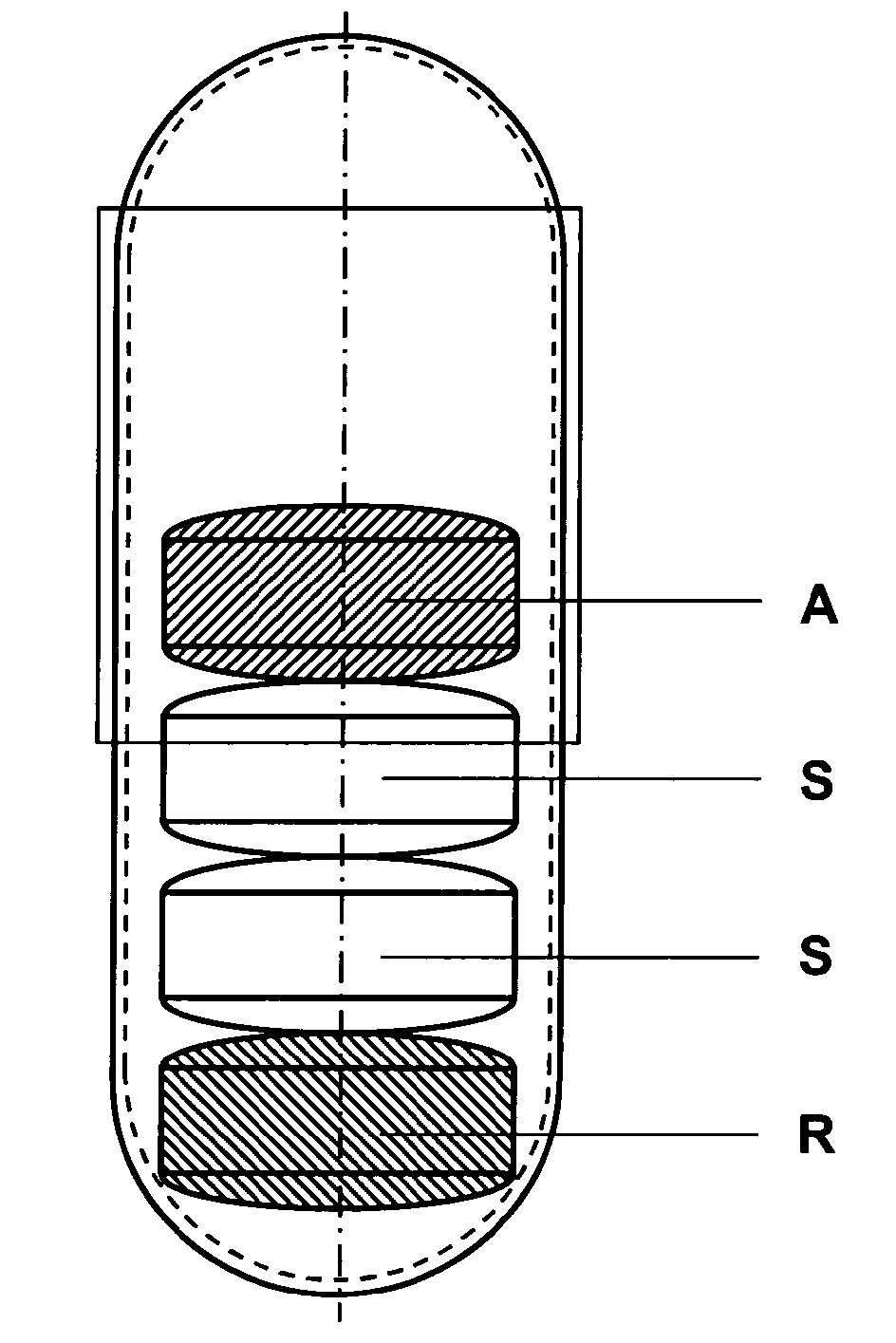
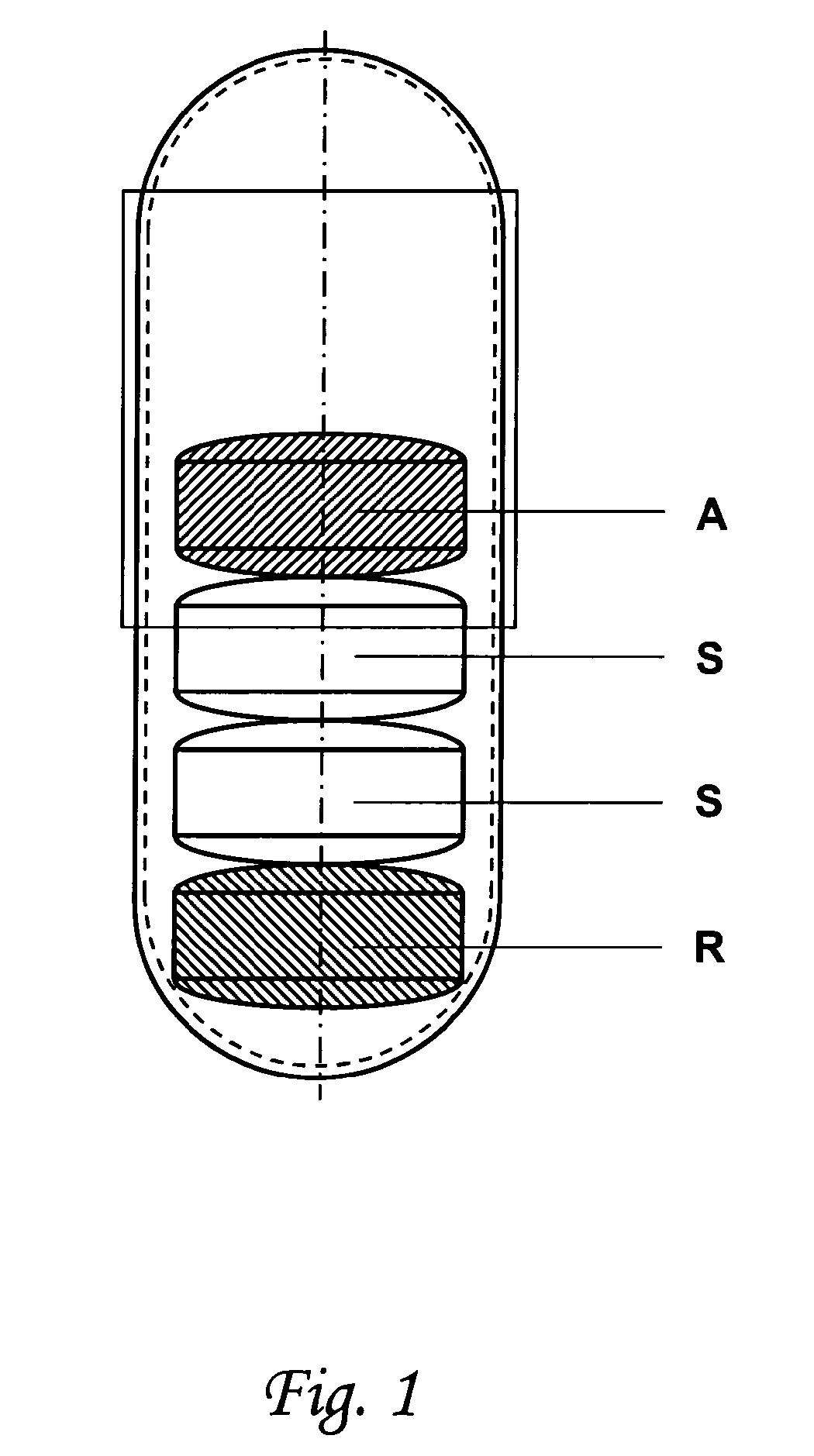
Capsule for the prevention of cardiovascular diseases
ActiveUS20110086094A1Improve the level ofLess impuritiesBiocideAntipyreticHigh risk populationsLisinopril
The invention relates to a capsule for the prevention of cardiovascular diseases which comprises coated tablets of acetylsalicylic acid, coated tablets of simvastatin or pravastatin, and coated tablets of lisinopril, ramiphl or perindopril. The capsules are used for the prevention of cardiovascular diseases in high-risk populations.
Owner:FERRER INT SA +1
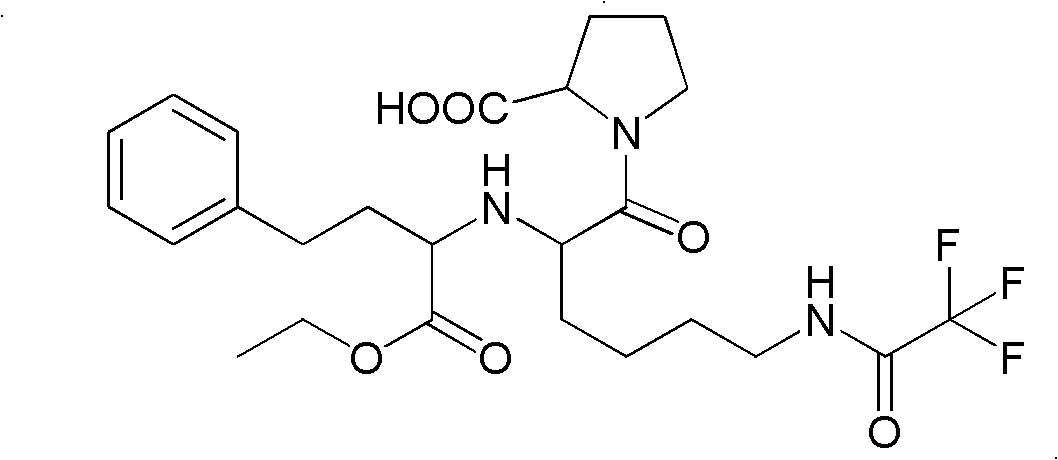
Precursor of Lisinopril compound and synthetic method
A precursor of lisinopril is prepared from activated alpha bromoacid and proline or its derivative through condensation to generate bipeptide, closing cycle in molecular under the action of strong alkali, opening lactone cycle by alkali in solvent to obtain the compound containing free hydroxy and carboxy, protecting or activating said two radicals, and condensating with high-benzene ethyl lactamate. Its advantages are high output rate and no pollution.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
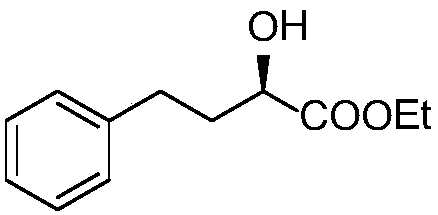
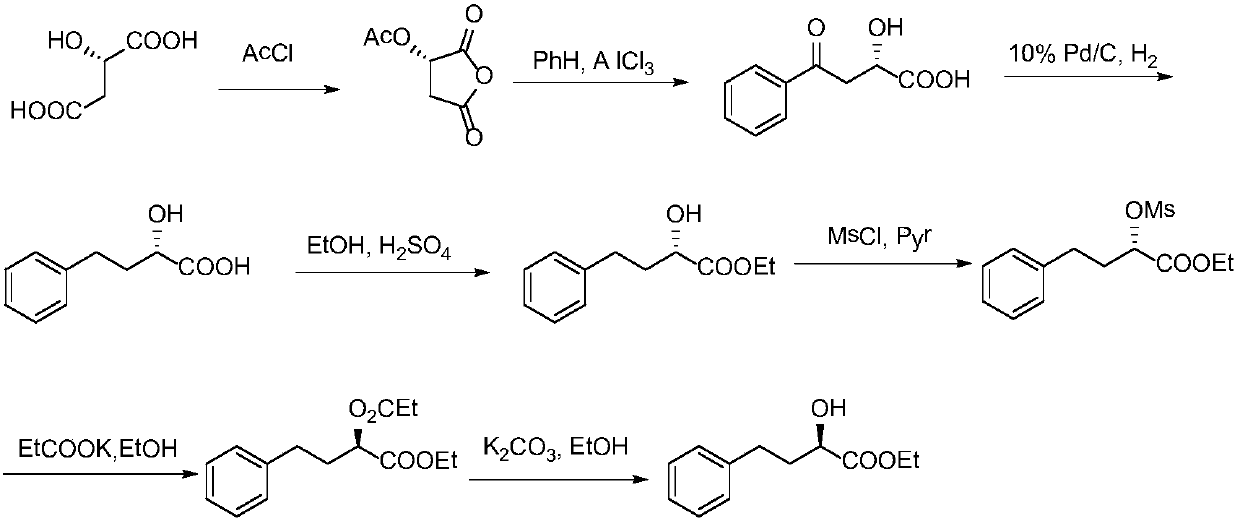
Method for preparing lisinopril intermediate
A method for preparing a lisinopril intermediate is provided. The method includes: treating (R)-hydroxy-4-phenylbutyrate with sulfonyl chloride in an organic solvent in the presence of a base to obtain a solution of sulfonate; reacting the obtained solution with a salt of trifluoroacetyl lysine; and obtaining a N2-[1-(S)-alkoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysine by separating after the reaction is completed. The method provided has a shorter synthesis route, is easy to operate, has a low cost, and is suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +2
Stable lisinopril tablet and preparation method thereof
InactiveCN104147588AAvoid bad consequencesImprove product qualityDipeptide ingredientsDrageesMoisture absorptionLisinopril
The invention belongs to the technical field of medicines, and in particular to a stable lisinopril tablet and a preparation method thereof. The stable lisinopril tablet is prepared by dry granulation, and is coated, so that a series of adverse consequences resulting from moisture absorption of the tablet can be avoided, and the product quality is more stable.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
Pharmaceutical composition containing lisinopril and amlodipine besylateand preparation method of pharmaceutical composition
InactiveCN103656608AReasonable prescriptionImprove stabilityOrganic active ingredientsDipeptide ingredientsCarboxymethyl starchAmlodipine besilate
The invention belongs to the technical field of medicines, and particularly relates to a pharmaceutical composition containing lisinopril and amlodipine besylate and a preparation method of the pharmaceutical composition. The pharmaceutical composition comprises the following components in parts by weight: 10 parts of lisinopril, 5 parts of amlodipine besylate (based on the weight of amlodipine), 100-200 parts of microcrystalline cellulose, 6-10 parts of sodium carboxymethyl starch, 1-3 parts of fumed silica and 2-4 parts of magnesium stearate. The pharmaceutical composition is prepared by using a dry granulating tableting process. The pharmaceutical composition has reasonable formula and good stability. The preparation method is simple in process and high in production efficiency; the preparation process of the pharmaceutical composition does not cause the increase of impurity content; the content uniformity of the prepared pharmaceutical composition is good.
Owner:HAINAN ZHONGJI MEDICAL TECH
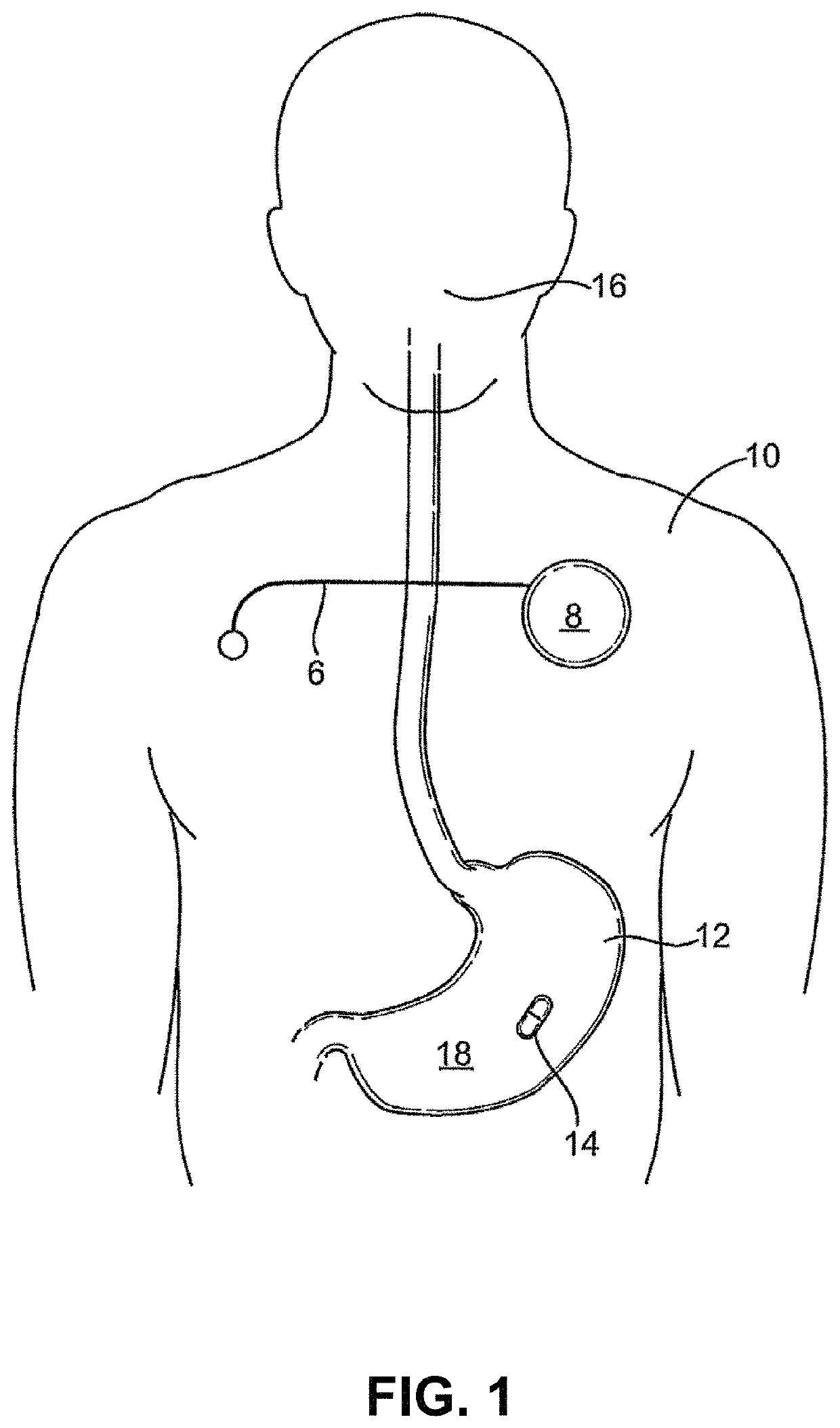
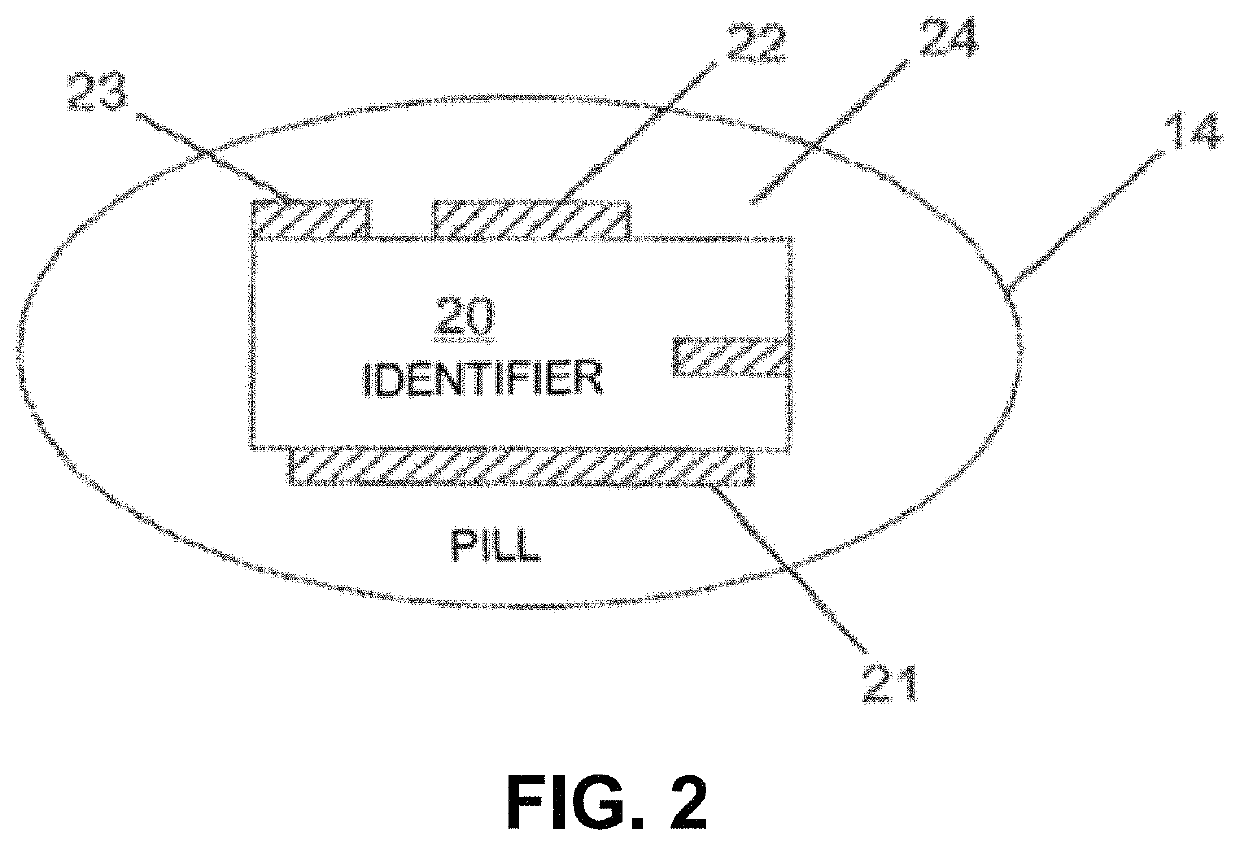
Lisinopril compositions with an ingestible event marker
Provided herein are compositions for the ingestible administration of lisinopril. In some embodiments the compositions comprise lisinopril and silicon. In some embodiments, the compositions comprise lisinopril, silicon, magnesium metal, and copper (I) chloride. Also provided herein are apparatuses comprising the compositions provided herein. Also provided herein are methods for using the compositions and apparatuses provided herein.
Owner:OTSUKA PHARM CO LTD
Sustained and controlled-release transdermal patch containing lisinopril
ActiveCN107028918ASmooth releaseGood reproducibilityDipeptide ingredientsPharmaceutical non-active ingredientsDrug reservoirControlled release
The invention discloses a sustained and controlled-release transdermal patch containing lisinopril, and relates to the field of pharmaceutical preparations. The transdermal patch comprises a drug reservoir layer, a backing layer and a protective layer, wherein the drug reservoir layer is composed of the lisinopril, a reservoir substrate and a penetration enhancer. The drug reservoir layer of each dosage of the sustained and controlled-release transdermal patch consists of 10-80mg of the lisinopril in terms of effective dose; and in percentage by weight, the drug reservoir layer consists of 2.5-25% of the lisinopril, 70-97% of the reservoir substrate and 0.5-5% of the penetration enhancer. The sustained and controlled-release transdermal patch containing the lisinopril provided by the invention, in comparison with lisinopril normal tablets, capsules or oral sustained-release preparations on the current market, is uniform in drug release, concrete in curative effect and stable in quality.
Owner:FERGUSON WUHAN BIOTECH
Medicinal composition containing amlodipine, ACE inhibitor and stains
InactiveCN101642571AAvoid drastic changesBalance blood pressureOrganic active ingredientsSenses disorderDiseaseImidapril
The invention relates to a medicinal composition. The composition comprises 1) any one of the following two ingredients: a) levamlodipine or pharmaceutically accepted salt thereof, or b) amlodipine orpharmaceutically accepted salt thereof; 2) ACE inhibitor (ACEI) or pharmaceutically accepted salt thereof; 3) stains or pharmaceutically accepted salt thereof; and 4) pharmaceutically accepted vector, wherein the amlodipine is selected from levamlodipine beaylate and amlodipine besylate; the ACEI is selected from lisinopril, quinapril, imidapril or pharmaceutically accepted salt thereof; and thestains is selected from atorvastatin, simvastatin, pravastatin or pharmaceutically accepted salt thereof. The composition is used for treating various high blood pressure accompanied with or not accompanied with hyperlipidaemia, angina and atherosclerosis, can treat or prevent heart cerebrovascular diseases related to high blood pressure, and improves the compliance of patient administration.
Owner:王丽燕
Refining method for lisinopril hydride
ActiveCN105439892AHigh purityLess impuritiesCarboxylic acid amide separation/purificationPeptidesKetone solventsFiltration
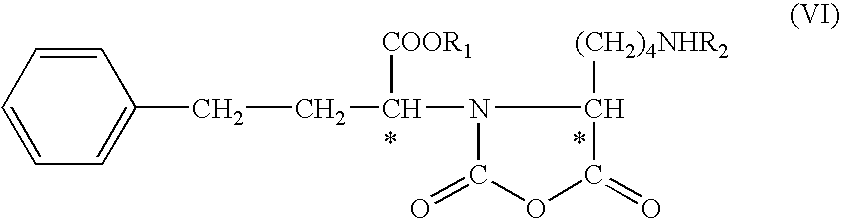
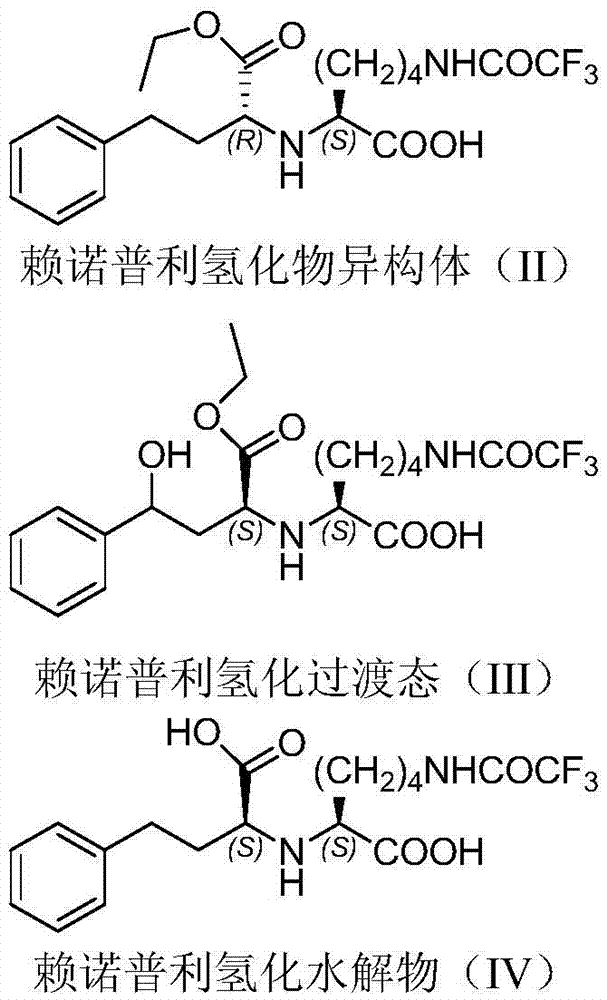
The invention provides a method for refining a crude lisinopril hydride product through solvent crystallization. The method comprises the following concrete steps: adding a ketone solvent into a crude product containing lisinopril hydride (I), lisinopril isomer (II) and other impurities (such as impurities III and IV); carrying out heating to dissolve the crude product; then carrying out cooling and crystallization for the first time; selectively adding a seed crystal and continuing cooling and crystallization; carrying out crystal growing and continuing cooling and crystallization; and then carrying out pumping filtration so as to obtain lisinopril hydride, wherein yield of lisinopril hydride reaches 80%, and purity of lisinopril hydride is more than 97%, or even more than 98%. The method can separate and refine hydride in the crude lisinopril hydride product, improves the refining yield of lisinopril hydride and has obvious utilization value in industrial application. The lisinopril hydride (I), the lisinopril isomer (II), a lisinopril hydrogenation transition state (III) and a lisinopril hydrogenation transition state (IV) are as described in the specification.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
A kind of preparation method of lisinopril intermediate
ActiveCN105777546BHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationBenzaldehydeDouble bond
The invention provides a method for preparing a lisinopril intermediate. The lisinopril intermediate is (R)-2-hydroxyl-4-phenylbutyrate. The method has the advantages that the lisinopril intermediate is made of inexpensive and easily available raw materials which are benzaldehyde and pyruvic acid, four-step efficient reaction including condensation, biological enzyme catalytic asymmetric reduction, double-bond hydrogenation and esterification is carried out on the benzaldehyde and the pyruvic acid, and accordingly an optically pure target product (R)-HPBE [(R)-2-hydroxyl-4-phenylbutyrate] can be ultimately obtained at the overall yield of 83%.
Owner:SUZHOU LEAD BIOTECH CO LTD
Lisinopril intermediate and purification method therefor
The invention relates to a lisinopril intermediate, i.e., (S)-2-((S)-2,5-dioxo-4-(4-(2,2,2-trifluoroacetylamino)butyl)oxazolidin-3-yl)-4-phenylethyl butyrate and a purification method therefor. The method comprises the following steps: adding toluene into a crude lisinopril anhydride intermediate under nitrogen protection, carrying out heating, stirring and dissolved clarification, then, adding aC5-10 alkane solvent, carrying out heat preservation and stirring, then, carrying out suction filtration, and carrying out baking, thereby obtaining a product I. According to the purification method,homophenylalanine impurities can be effectively removed, total impurities are also reduced, the finished product quality is improved, and high-quality lisinopril can be stably obtained through processproduction.
Owner:SHANGHAI SYNCORES TECH INC +1
Compound ramipril nano-emulsion for antihypertension
InactiveCN102423483AEvenly distributedSystem transparencyOrganic active ingredientsDipeptide ingredientsAdjuvantActive agent
The invention discloses an oil-in-water type compound ramipril nano-emulsion, which is prepared from 1%-25% of ramipril, 0.1%-25% of hydralazine, 0.1%-25% of lisinopril, 10%-55% of surfactant, 5%-30% of oil and the balance distilled water, wherein the sum of the mass percent of the ingredients is 100%. The nano-emulsion is small in emulsion droplet granule, even in distribution, low in viscosity, and good in liquidity and has antihypertensive capacity, the dissolubility of the ramipril is enhanced, the bioavailability and the drug stability of the ramipril are improved, the metabolic time of the nano-emulsion in vivo is delayed, and the problem of hypertension can be synthetically solved because the hydralazine and the lisinopril are synthesized. With the adoption of the compound ramipril nano-emulsion, the dosage of the adjuvants of the antihypertension is reduced, the toxicity of the antihypertension to body is lowered, and the production cost is lowered; and furthermore, the preparation method of the compound ramipril nano-emulsion is simple and is low in energy consumption.
Owner:NORTHWEST A & F UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka.patsnap.com/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C1.PNG)
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka.patsnap.com/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C2.PNG)
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka.patsnap.com/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C3.PNG)