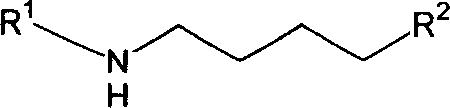
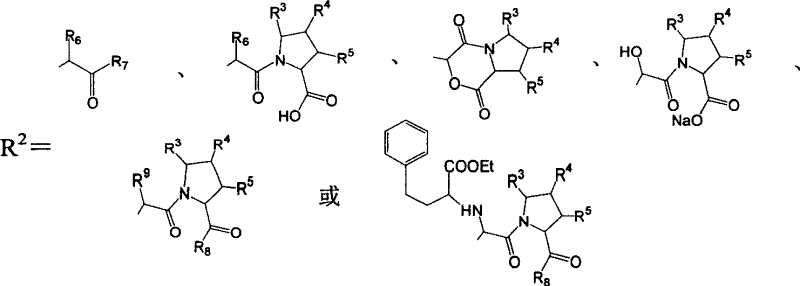
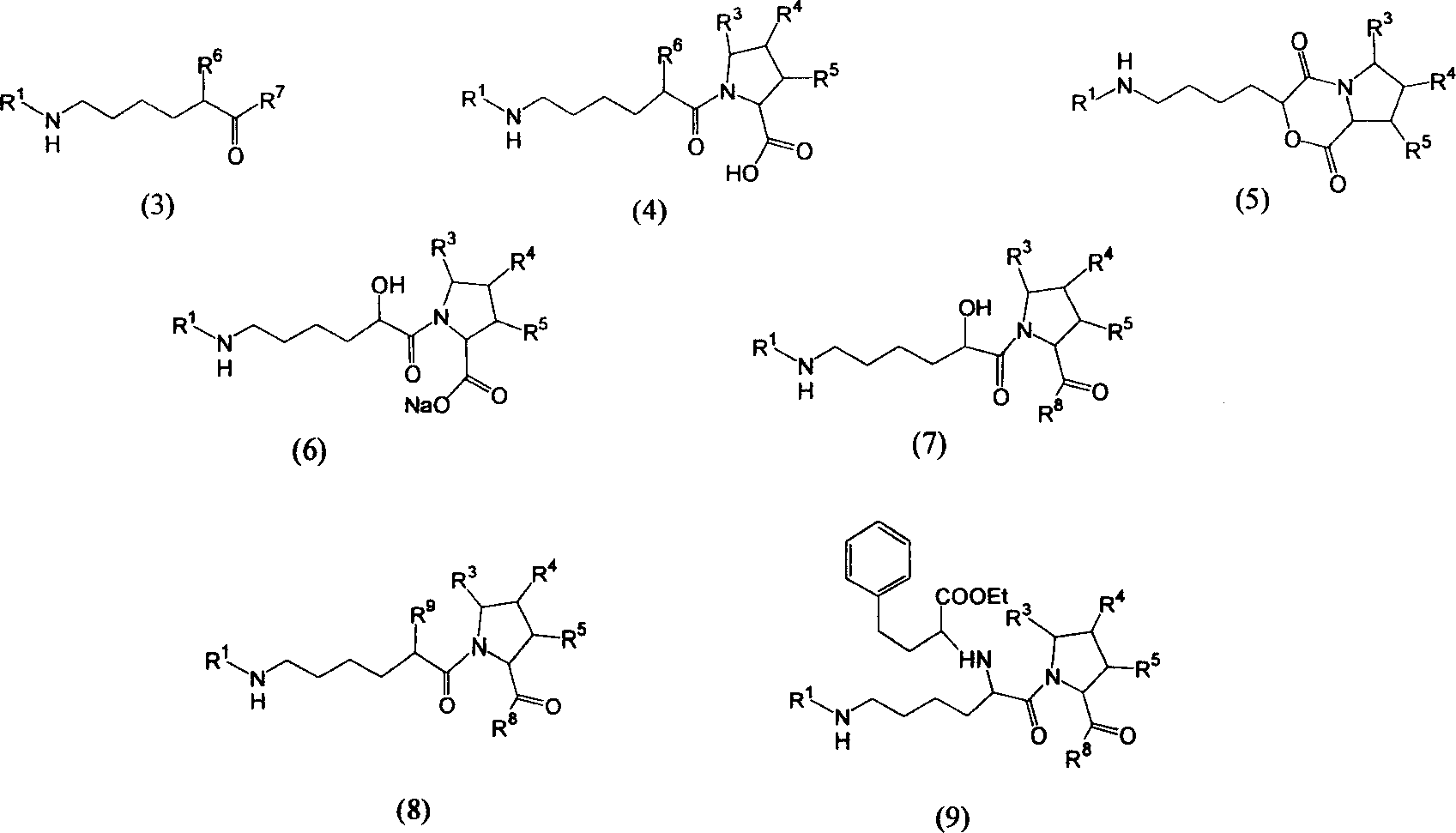
Precursor of Lisinopril compound and synthetic method
A synthesis method and compound technology are applied to the precursors of lisinopril compounds and the field of synthesis thereof, and can solve problems such as unfavorable factors in industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: diazotization
[0049] 0.01mol of lysine trifluoroacetate and 0.035mol of potassium bromide were dissolved in 2.5N sulfuric acid (0.052mol), cooled to -5°C in an ice-salt bath, and the mixture was added dropwise with 0.01-0.02mol of nitrous acid under stirring Sodium aqueous solution, after the dropwise addition, the temperature rises naturally, stirs at room temperature for about 5 hours (overnight), stops stirring, and stands still, the yellow oil and water are separated, the upper layer of water is poured off, and after post-treatment, ethyl acetate The extract was washed with brine, dried over anhydrous sodium sulfate, and concentrated to obtain a colorless oily liquid with a yield of 86%.
[0050] The spectrogram data is as follows:
[0051] H-NMR: CDCL3, δ7.2 (NH, S, 1H); δ3.4 (NCH 2 , t, 2H); δ4.3(BrCH, m, 1H) δ1.6-2.2(CH 2 CH 2 CH 2 , m, 6H)
[0052] MS: M 306
[0053] Optical rotation: CHCl 3 , c=1, α=-18.5°
Embodiment 2
[0054] Embodiment 2: condensation reaction
[0055] Dissolve 0.1 mol of bromic acid generated in the first step in CH 2 Cl 2 0.11 mol of thionyl chloride was added dropwise in an ice-water bath. After the dropwise addition, it was changed to a reflux device and refluxed for 4 hours.
[0056] Dissolve 0.2 mol of proline in an appropriate amount of chloroform, add dropwise the chloroform solution of 0.1 mol of the above-mentioned acid chloride under stirring, stir overnight for reaction, post-treatment, and recrystallize to obtain the compound Yield 95%.
[0057] H-NMR: CDCl 3 δ7.16 (CONH, S, 1H); δ4.85 (BrCH, m, 1H); δ4.30 (CHCOOH, m, 1H); δ3.80-3.58 (5'-CH 2 , m, 2H); δ3.42-3.25 (NHCH 2 , m, 2H); δ2.40-1.95 (3'-CH 2 , 4'-CH 2 , 3-CH 2 , m, 6H); δ1.70-1.30 (4-CH 2 ,5-CH 2 , m, 4H).
[0058] MS: ESI, M403
Embodiment 3
[0059] Embodiment 3: ring closing reaction
[0060] compound Dissolve in DMF, add 0.1mol sodium bicarbonate under stirring, react until TLC detects that there is no raw material, post-treatment, dissolve in ethyl acetate, wash with water, wash with brine, dry with anhydrous sodium sulfate, concentrate, and recrystallize to obtain the ring-closed product Compound, yield 86%.
[0061] H-NMR: CDCl 3 δ7.00-6.80 (NH, S, 1H); δ4.80 (3-CH, t, 1H); δ4.25 (6-CH, t, 1H); δ3.60 (9-CH 2 , m, 2H); δ3.4(NHCH 2 , m, 2H); δ2.40-1.60 (1'-CH 2 , 2'-CH 2 , 3’-CH 2 ,7-CH 2 ,8-CH 2 , m, 10H).
[0062]
[0063] ESI: M+1 323
[0064] Optical rotation value: CHCl3, c=1.0035, α=-110.89°
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com