Method for preparing lisinopril intermediate
An intermediate, lysine technology, applied in the field of preparation of lisinopril intermediates, can solve the problems of high safety protection cost, easy spontaneous combustion, low product yield, etc., to avoid toxic by-products, increase content, reduce cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
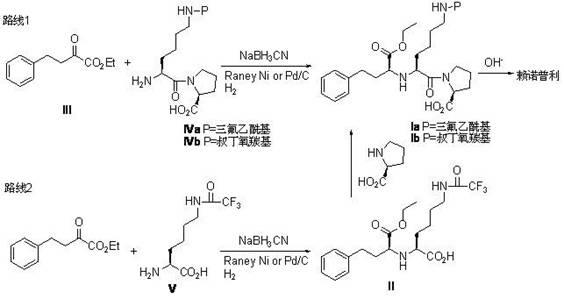
[0022] This embodiment adopts the following steps:
[0023] 32.4g (0.095mol) N e -(trifluoroacetyl)-L-lysine-L-proline with 23.05g (0.109mol) NaBH(OAc) 3 , 5.1ml (0.088mol) glacial acetic acid, 200ml 1,2-dichloroethane mixed, stirred well; cooled to 5 oC , then dropwise add a mixture of 19.6g (0.096mol) ethyl α-oxophenylbutyrate dissolved in 25ml 1,2-dichloroethane, drop it for about 30min, and then in 10 oC React at room temperature for 1 h, then react at room temperature for 4 h, TLC (thin layer chromatography) to detect the reaction end point; add 100 ml of water, separate the liquids, and extract the aqueous phase with dichloroethane (100ml×2), each time with dichloroethane 100 ml of alkanes, extracted twice, combined the organic phases, dried with anhydrous sodium sulfate, removed the solvent, and recrystallized the residue with methyl tert-butyl ether / n-hexane to obtain 35.8 g of white crystals, yield 71.2%, m.p.75.3 -77.4 oC , [a] 25 405 -53.8 o [a] 25 D ...
Embodiment 2
[0029] N e -(tert-butoxycarbonyl)-N a -Synthesis of [(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-lysine-L-proline (Ⅰb)
[0030] This embodiment includes the following steps:
[0031] 16.29g (47.5mmol) N e -(tert-butoxycarbonyl)-L-lysine-L-proline with 21.57g (54.5mmol) NaBH(OAc) 3 , 2.6ml (44.7mmol) glacial acetic acid, 100ml 1,2-dichloroethane mixed, stirred evenly; cooled to 5 oC , and then dropwise added a mixture of 9.8g (48mmol) ethyl α-oxobenzenebutyrate dissolved in 15ml 1,2 dichloroethane, and dropped it in about 20 minutes. oCReact at room temperature for 30 minutes, then react at room temperature for 4 hours, TLC to detect the end point, add 50ml of water, separate the liquids, extract the aqueous phase with dichloroethane (50ml×2), combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent. The residue was recrystallized from ethyl acetate / n-hexane to obtain 17.4g, yield 68.5%, white crystal, m.p. 81.3-83.1 oC .
[0032] The N prepa...
Embodiment 3
[0034] N e -(Trifluoroacetyl)-N a -Synthesis of [(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-lysine-L-proline (Ⅰa)
[0035] This embodiment adopts the following steps:
[0036] 16.2g (47.5mmol) N e -(trifluoroacetyl)-L-lysine-L-proline, 9.9g (47.9mmol) ethyl a-oxophenylbutyrate, 2.6ml glacial acetic acid (44.7mmol) and 150ml dichloromethane were mixed , stir well; cool to 5 oC , then dropwise added 11.6g (55mmol) NaBH(OAc) 3 Dissolve the mixed solution in 75ml of dichloromethane solution, drop it in about 30min, in 10 oC React at low temperature for 30 minutes, then warm up to room temperature and react for 2.5 hours, and detect the end point by TLC; after the reaction is completed, add 50ml of water, separate the liquids, extract the aqueous phase with dichloromethane (50×2ml), combine the organic phases, and dry them with anhydrous sodium sulfate. The solvent was removed, and the residue was recrystallized from methyl tert-butyl ether / n-hexane to obtain 17.6 g of white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com