Lisinopril controlled-release tablet and preparation method thereof
A slow-release tablet and delayed-release technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, drug combinations, etc., can solve the problems of complex preparation process, affecting the stability of preparations, and many coating ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
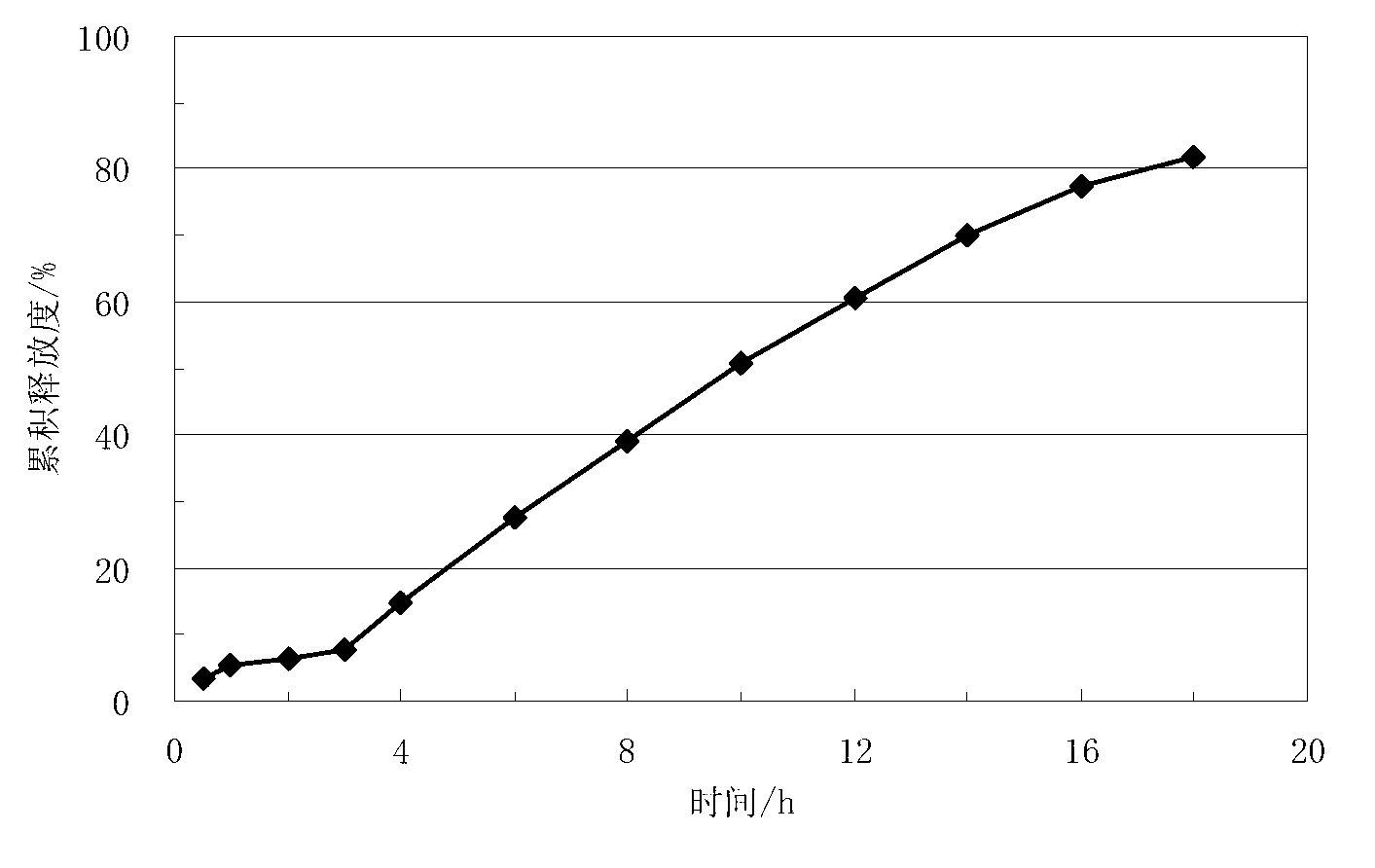
Embodiment 1
[0045] Lisinopril delayed-release sustained-release tablets, which are composed of a core containing lisinopril and a coating layer wrapped around the core. The weight of each tablet core is 200 mg. The coating layer The weight gain is 8.7mg, calculated based on the total weight of the tablet, the coating layer accounts for 4.2% of the total weight of the tablet. In this embodiment, the dosage of the following prescriptions containing lisinopril-containing tablet cores and coating layers is calculated according to the amount of 1500 tablets, and the prescription of the described tablet cores containing lisinopril is as follows:
[0046] Lisinopril: 45g;
[0047] Lactose: 120g;
[0048] Hydroxypropyl methylcellulose (HPMC K4M): 120g;
[0049] Polyvinylpyrrolidone (PVP): 9.0g;
[0050] Magnesium stearate: 3.0g;
[0051] Micronized silica gel: 3.0g.
[0052] The prescription of the coating layer is as follows:
[0053] Ethyl cellulose: 10g;
[0054] Polyethylene glycol: 3.0g.
[0055] The pre...
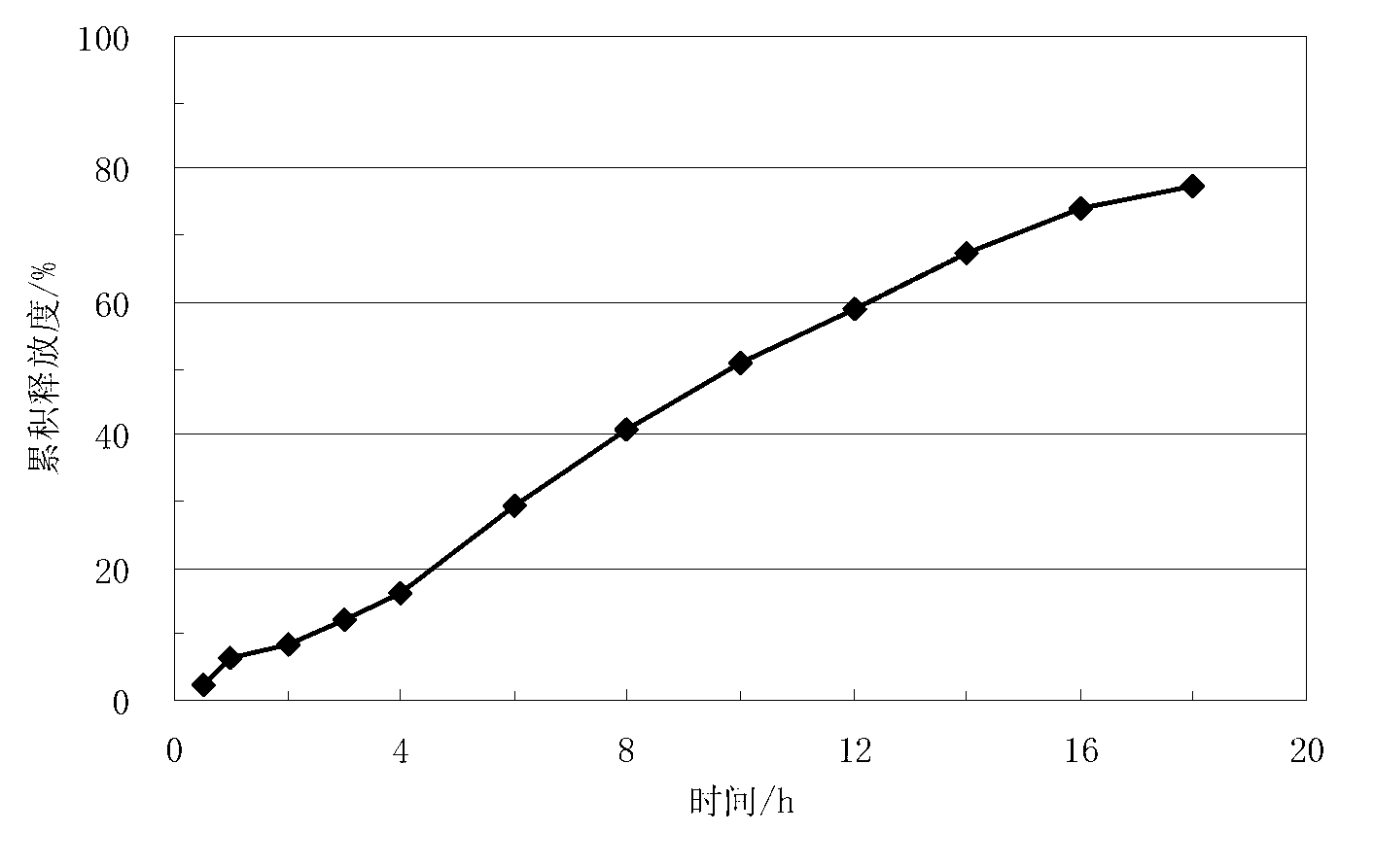
Embodiment 2
[0060] Lisinopril delayed-release sustained-release tablets, which are composed of a core containing lisinopril and a coating layer wrapped around the core. The weight of each tablet core is 200 mg. The coating layer The weight gain is 9.3mg, calculated based on the total weight of the tablet, the coating layer accounts for 4.4% of the total weight of the tablet. In this embodiment, the dosage of the following prescriptions containing lisinopril-containing tablet cores and coating layers is calculated according to the amount of 1500 tablets, and the prescription of the described tablet cores containing lisinopril is as follows:
[0061] Lisinopril: 45g;
[0062] Microcrystalline cellulose: 75g;
[0063] Hydroxypropyl methylcellulose (HPMC K4M): 165g;
[0064] Polyvinylpyrrolidone (PVP): 9.0g;
[0065] Magnesium stearate: 3.0g;
[0066] Micronized silica gel: 3.0g.
[0067] The prescription of the coating layer is as follows:
[0068] Ethyl cellulose: 10g;
[0069] Polyethylene glycol: 4.0...
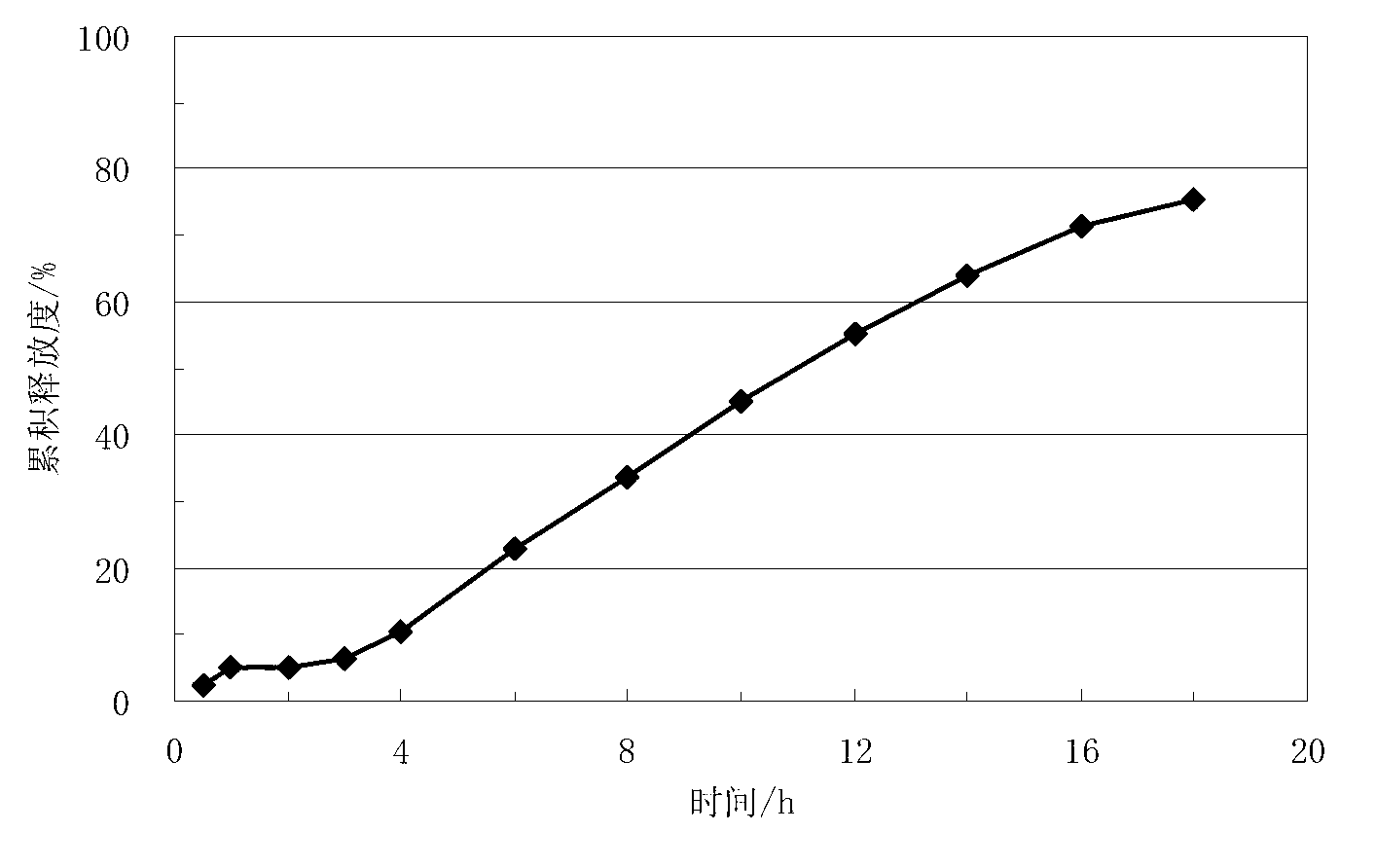
Embodiment 3
[0075] Lisinopril delayed-release sustained-release tablets, which are composed of a core containing lisinopril and a coating layer wrapped around the core. The weight of each tablet core is 200 mg. The coating layer The weight gain is 9.3mg, calculated based on the total weight of the tablet, the coating layer accounts for 4.4% of the total weight of the tablet. In this embodiment, the dosage of the following prescriptions containing lisinopril-containing tablet cores and coating layers is calculated according to the amount of 1500 tablets, and the prescription of the described tablet cores containing lisinopril is as follows:
[0076] Lisinopril: 45g; starch: 120g; hydroxypropyl methylcellulose (HPMC K15M): 120g; ethyl cellulose: 9.0g; magnesium stearate: 3.0g; micronized silica gel: 3.0g.
[0077] The prescription of the coating layer is as follows:
[0078] Cellulose acetate: 10g; polyethylene glycol: 4.0g.
[0079] The preparation method of lisinopril delayed-release sustained-r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com