A kind of preparation method of lisinopril intermediate
An intermediate and hydroxyl technology, which is applied in the field of preparation of lisinopril intermediates, can solve the problems of high refining difficulty, high cost, and low yield, and achieve the effects of environmental friendliness, mild conditions, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
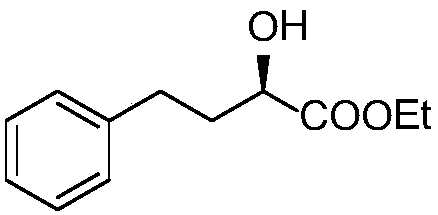
[0043] The lisinopril intermediate prepared in this example is (R)-2-hydroxy-4-phenylbutyric acid ethyl
[0044]
[0045] Esters have the following structure:
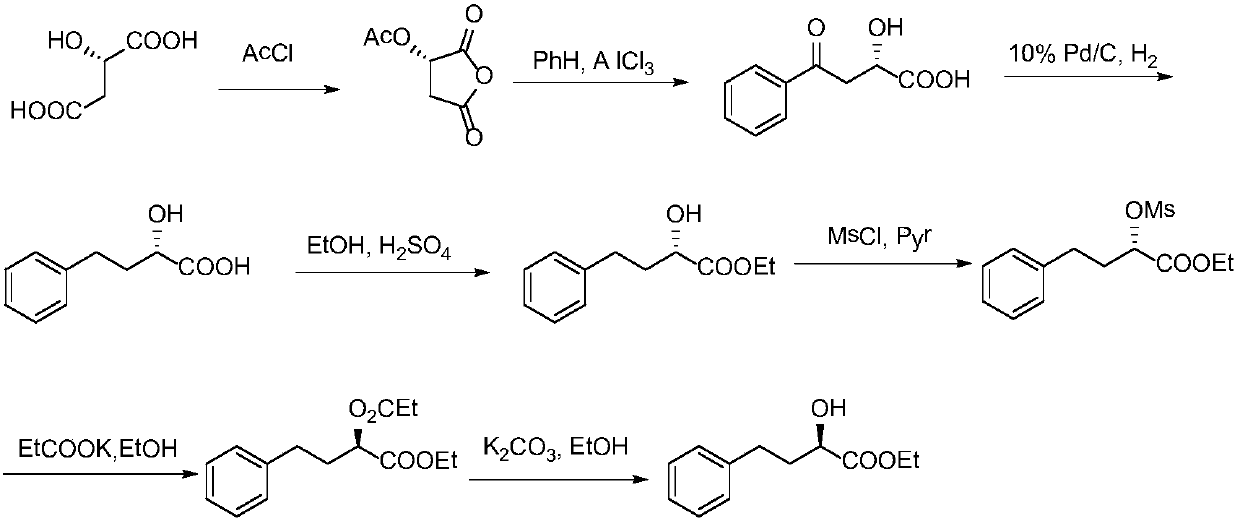
[0046] The above-mentioned ethyl (R)-2-hydroxy-4-phenylbutyrate uses cheap and easy-to-obtain benzaldehyde and pyruvic acid as raw materials, through condensation, biological enzyme-catalyzed asymmetric reduction, double bond hydrogenation and esterification in four efficient steps Reactive synthesis, the synthetic route is as follows:
[0047]
[0048] Its preparation method is specifically:
[0049] (1) Under a nitrogen atmosphere, 21.2 g of benzaldehyde was dissolved in 15 mL of methanol, the reaction system was lowered to 0°C and 17.2 g of pyruvic acid was added while maintaining this temperature. The methanol solution of potassium hydroxide (16.8g potassium hydroxide is dissolved in the methanol of 60mL) is slowly added dropwise, and the temperature of the reaction system is controlled below 15°C. After t...
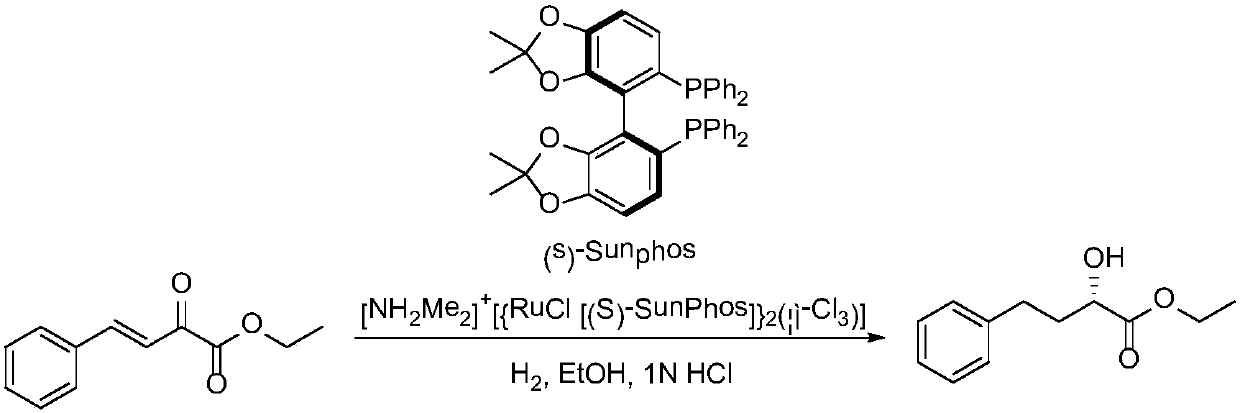
Embodiment 2
[0054] This example is the same as the preparation method of (R)-2-hydroxy-4-phenylbutyric acid ethyl ester described in Example 1, the only difference is that step (2) is: take the product obtained in the above step 1) Add 10 g of potassium unsaturated ketoacid and 20 mL of isopropanol to a 250 mL reactor filled with 80 mL of 0.2M PBS buffer at pH=6.0 and stir evenly, then add 6N hydrochloric acid dropwise to adjust the pH of the system to 6.0. Add 0.25 g of ketoreductase powder (purchased from Suzhou Pivot Biotechnology Co., Ltd.: Product No. YHADH047) and NADP+0.01 g in sequence, and stir at 30° C. for 24 hours. The reaction conversion rate is >92% as monitored by HPLC. After the reaction was completed, 6N hydrochloric acid was slowly added dropwise to the reaction system to adjust the pH to 1, and 50 mL of ethyl acetate was added to stir for 0.5 hour, filtered, the filtrate was separated into layers, and the organic phase was taken. The aqueous phase was extracted twice wi...
Embodiment 3
[0056] The preparation method of (R)-2-hydroxyl-4-phenylbutyric acid ethyl ester of the present embodiment may further comprise the steps:
[0057] (1) Under an inert gas, dissolve benzaldehyde in an organic solvent, add pyruvic acid at a temperature of 0°C, keep the temperature at 15°C, slowly drop a strong alkali methanol solution, and keep the temperature at 20°C after the addition is complete , stirred and reacted for 12h, after the reaction was completed, the reaction solution was filtered, and the obtained yellow solid was washed with methanol and ether to obtain the unsaturated keto acid;
[0058] Wherein, the organic solvent is methanol; the inert gas is nitrogen; the strong base is potassium hydroxide;
[0059] It should be noted that, as an alternative implementation of this embodiment, the above-mentioned 0°C can be replaced by any value between -5°C and 5°C; the above-mentioned 15°C can also be any value lower than 15°C; the above-mentioned 20°C can be replaced by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com