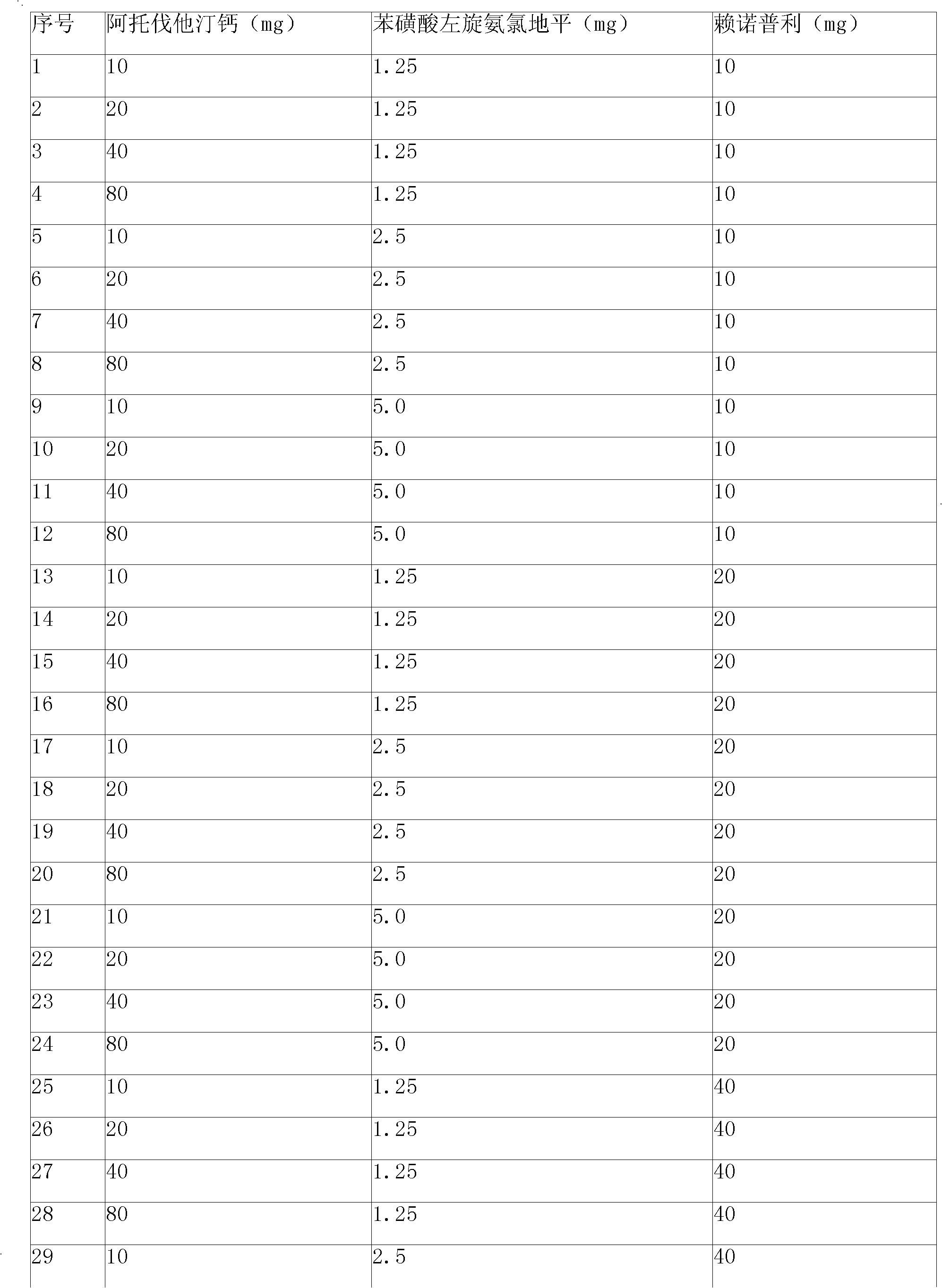Medicinal composition containing amlodipine, ACE inhibitor and stains
A technology of amlodipine and levamlodipine, which is applied in the field of medicine and can solve unsatisfactory problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
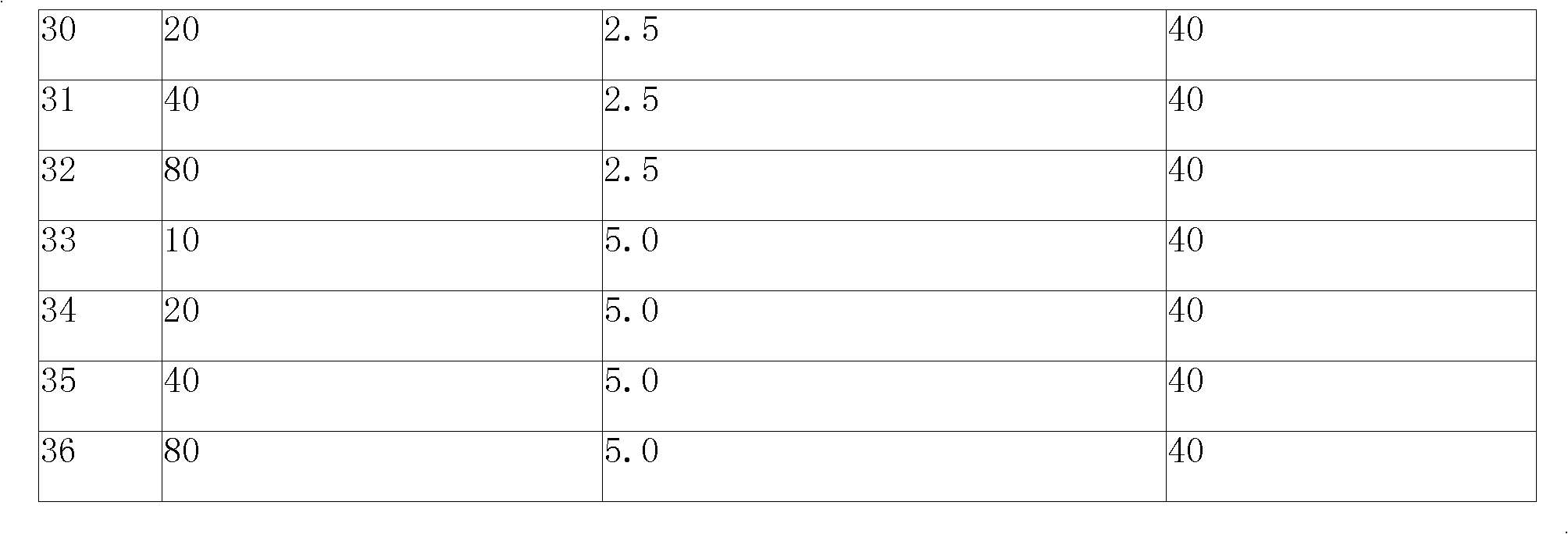
[0107] Embodiment 1: Levoamlodipine besylate, lisinopril and atorvastatin calcium tablet
[0108]
[0109]
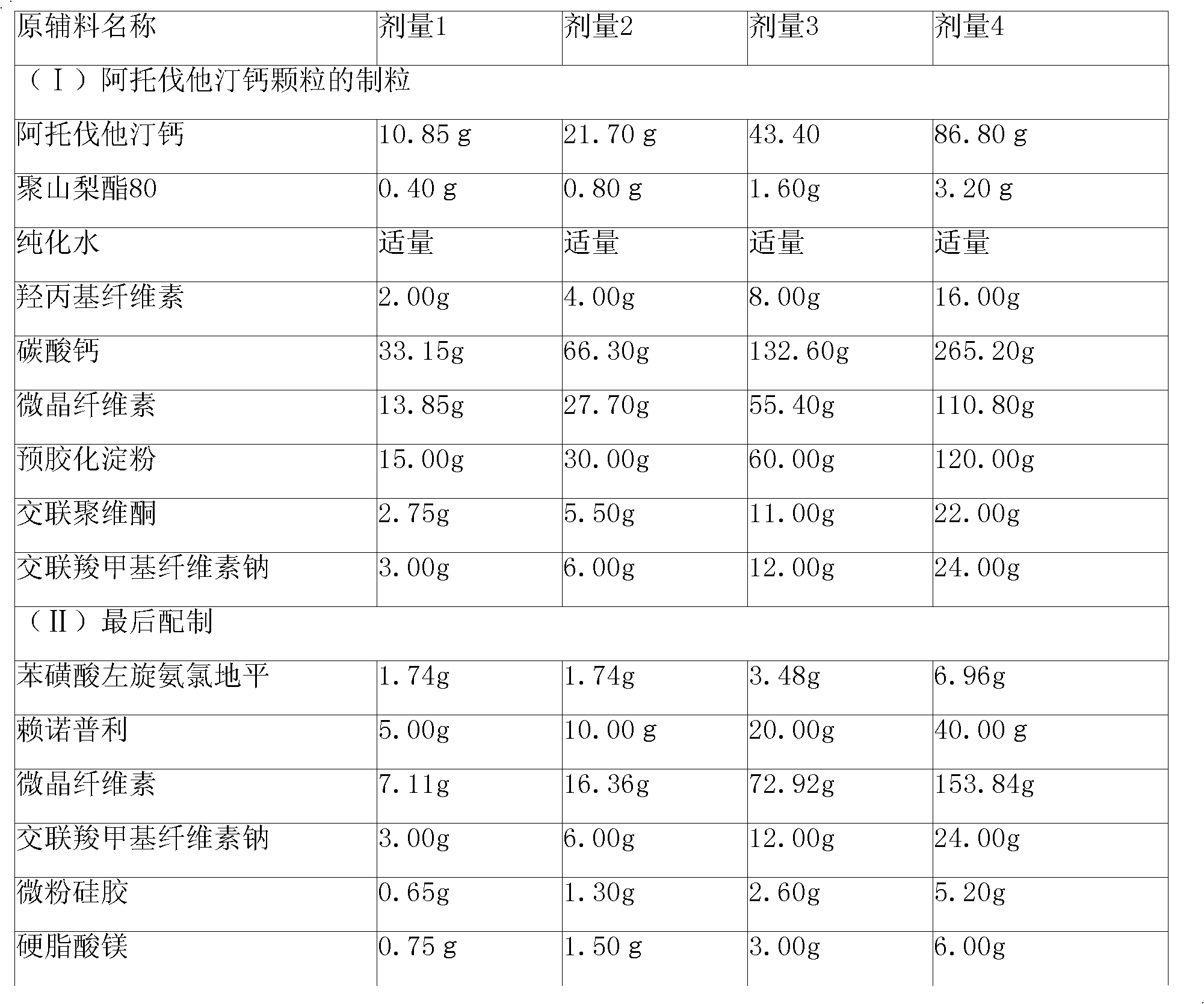
[0110] Preparation:
[0111] (1) Granulation of atorvastatin calcium granules
[0112] Step 1. Pass various solid raw and auxiliary materials through No. 5 to No. 6 sieves, and set aside;
[0113] Step 2, dissolving polysorbate 80 in purified water at 45°C to 60°C, adding hydroxypropyl cellulose, and cooling the solution to room temperature;
[0114] Step 3, mixing atorvastatin calcium, calcium carbonate, microcrystalline cellulose, precrosslinked starch, crospovidone and croscarmellose sodium in a granulator;
[0115] Step 4. Mix the powder mixture from step 3 and the solution from step 2 in the granulator, and stir while adding to make a suitable soft material. If necessary, adjust its pH to 5.5-10.0. Use No. 2 Sieve to make wet granules;
[0116] Step 5, dry the granules in the drying equipment, sieve the granules with No. 2 sieve after drying, and finally ...
Embodiment 2
[0122] Embodiment 2: levamlodipine besylate, quinapril hydrochloride and simvastatin capsules
[0123]
[0124]
[0125] Preparation:
[0126] (1) Granulation of Simvastatin Granules
[0127] Step 1. Pass various solid raw and auxiliary materials through No. 5 to No. 6 sieves, and set aside;
[0128]Step 2, dissolving polysorbate 80 in purified water at 50°C, adding hydroxypropyl cellulose, and cooling the solution to room temperature;
[0129] Step 3, mixing simvastatin, calcium carbonate, microcrystalline cellulose, precrossified starch, sodium lauryl sulfate and croscarmellose sodium in a granulator;
[0130] Step 4. Mix the powder mixture from step 3 and the solution from step 2 in the granulator, and stir while adding to make a suitable soft material, and adjust its pH value to 5.5-10.0 if necessary; use No. 2 Sieve to make wet granules;
[0131] Step 5, dry the granules in the drying equipment, sieve the granules with No. 2 sieve after drying, and finally make ...
Embodiment 3
[0137] Embodiment 3: Amlodipine besylate, imidapril hydrochloride and pitavastatin calcium dispersible tablets
[0138]
[0139] Preparation:
[0140] (1) preparation of pitavastatin calcium microcapsules
[0141] Step A, pass various solid raw and auxiliary materials through No. 5 to No. 6 sieves respectively, and set aside;
[0142] Step B. Dissolve gelatin and gum arabic in purified water respectively, stir to make them fully dissolved, add pitavastatin calcium and cross-linked carboxymethyl cellulose sodium to gum arabic, and ultrasonically emulsify for 45 minutes, and gelatin solution and gum arabic Mix the gum solution into a three-necked flask, control the stirring speed to 200-400rpm, heat in a water bath, keep the temperature at 45°C-50°C, adjust the pH value of the system to 3.5-4.0, react for 55 minutes, and lower the temperature of the system to 2°C -8°C, add formaldehyde with a mass concentration of 25% and a glutaraldehyde solution with a mass concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com