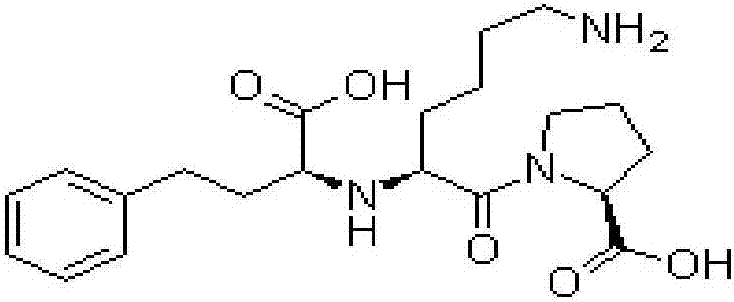
Sustained and controlled-release transdermal patch containing lisinopril
A technology of transdermal patch and penetration enhancer, which is applied in the field of pharmaceutical preparations, can solve problems such as difficult control of process-related technical parameters, poor drug compliance, and unstable release rate, so as to avoid the first-pass effect and prolong blood drug production. Concentration stable period, the effect of improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
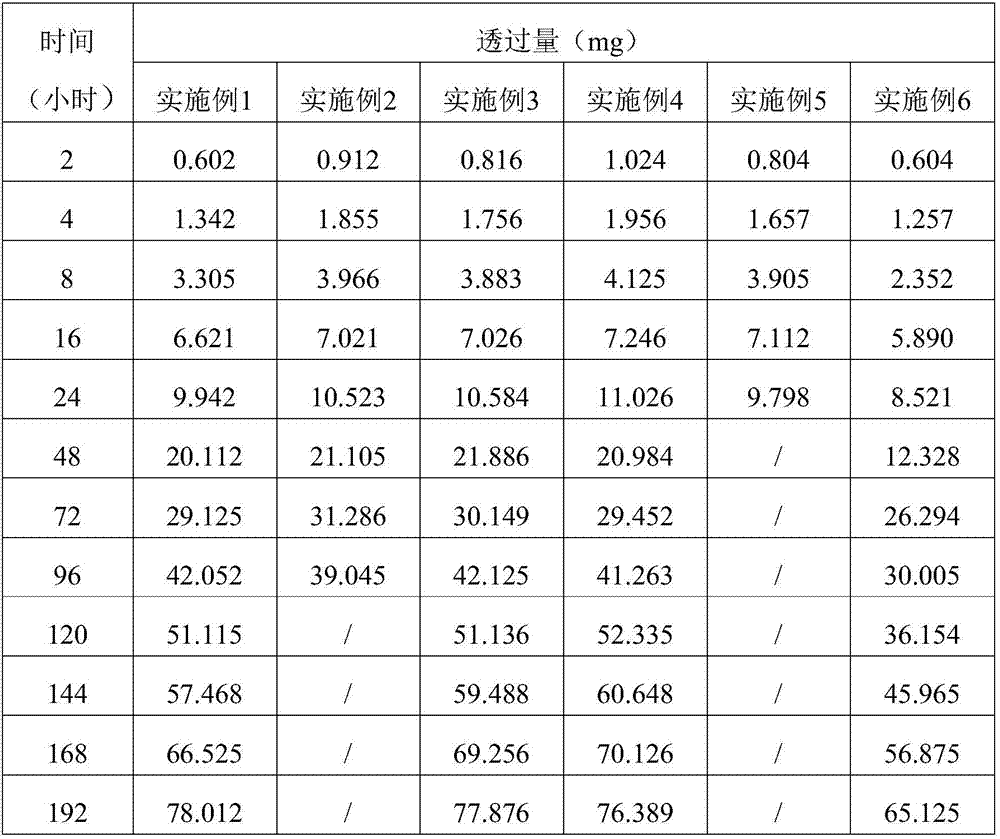
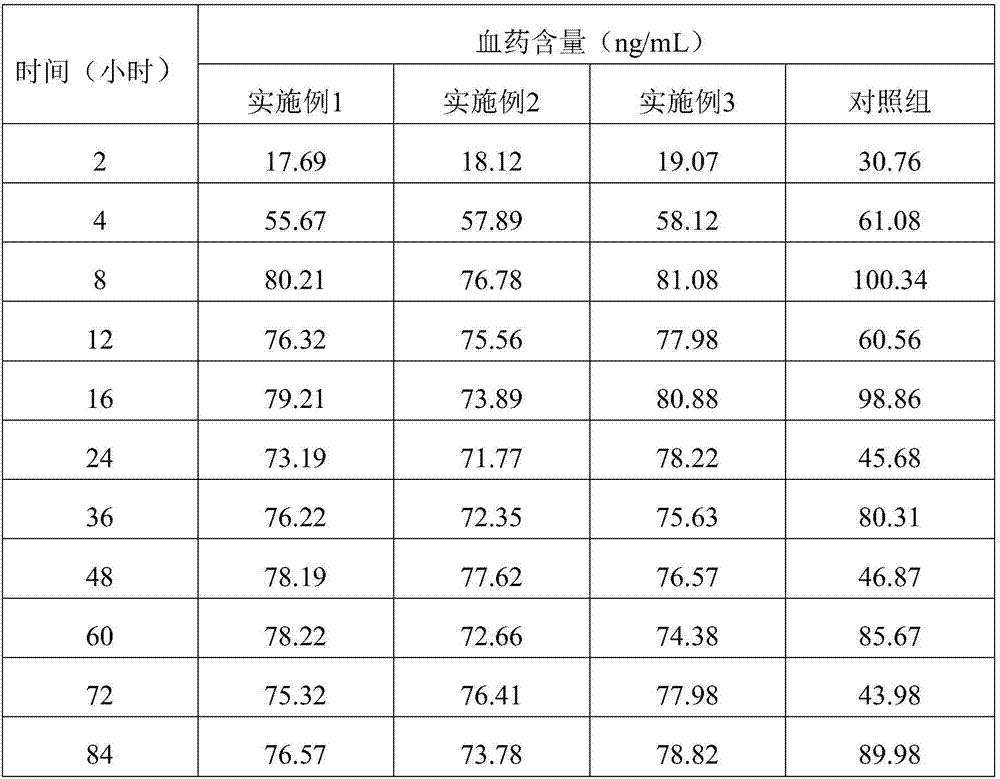
[0038] The embodiment of the present invention provides a sustained and controlled release transdermal patch containing lisinopril, comprising a drug reservoir layer, a backing layer and a protective layer, the drug reservoir layer is composed of lisinopril, reservoir matrix and Permeation enhancer composition, the sustained and controlled release transdermal patch containing lisinopril contained in each patch contains effective dose of lisinopril 80mg, by mass percentage, the drug reservoir layer contains 16 mg of lisinopril %, storage matrix 80%, penetration enhancer 4%, the content of the storage matrix in the drug storage layer is 100mg / cm 2 .
[0039] The drug reservoir layer of the transdermal patch in this example contains 80 g of lisinopril, 400 g of polyisobutylene pressure-sensitive adhesive, 10 g of lecithin, and 10 g of azone per 1000 patches. The backing layer is polyvinyl chloride and the protective layer is polyethylene.
Embodiment 2
[0041] The embodiment of the present invention provides a sustained and controlled release transdermal patch containing lisinopril, comprising a drug reservoir layer, a backing layer, a protective layer and an adhesive layer, the drug reservoir layer is composed of lisinopril, reservoir Reservoir matrix and permeation enhancer composition, the sustained and controlled release transdermal patch containing lisinopril contained in each paste contains effective dose of lisinopril 40mg, by mass percentage, the drug reservoir layer contains lisinopril 10% of Puli, 87.75% of reservoir matrix, 2.25% of penetration enhancer, the content of reservoir matrix in drug reservoir layer is 87.75mg / cm 2 .
[0042]Every 1000 pastes in the drug reservoir layer of the transdermal patch in this embodiment contain: lisinopril 40g, silicone pressure-sensitive adhesive 320g, carbomer 15g, triethanolamine 16g, lecithin 4.5g, azone 4.5g . The backing layer is non-woven fabric, the protective layer is...
Embodiment 3
[0044] The embodiment of the present invention provides a sustained and controlled release transdermal patch containing lisinopril, comprising a drug reservoir layer, a backing layer, and a protective layer, and the drug reservoir layer is composed of lisinopril, reservoir matrix and Penetration enhancer composition, the sustained and controlled release transdermal patch containing lisinopril described in each paste contains the lisinopril 80mg of effective dosage, by mass percentage, contains lisinopril 25mg in the described drug storage layer %, storage matrix 70%, penetration enhancer 5%, the content of the storage matrix in the drug storage layer is 50mg / cm 2 .
[0045] Every 1000 pastes in the drug reservoir layer of the transdermal patch in this embodiment contain: lisinopril 80g, silicone pressure-sensitive adhesive 287g, povidone 5g, methylcellulose 8g, lecithin 12g, azone 8g . The backing layer is polyester film, and the protective layer is polytetrachlorethylene fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com