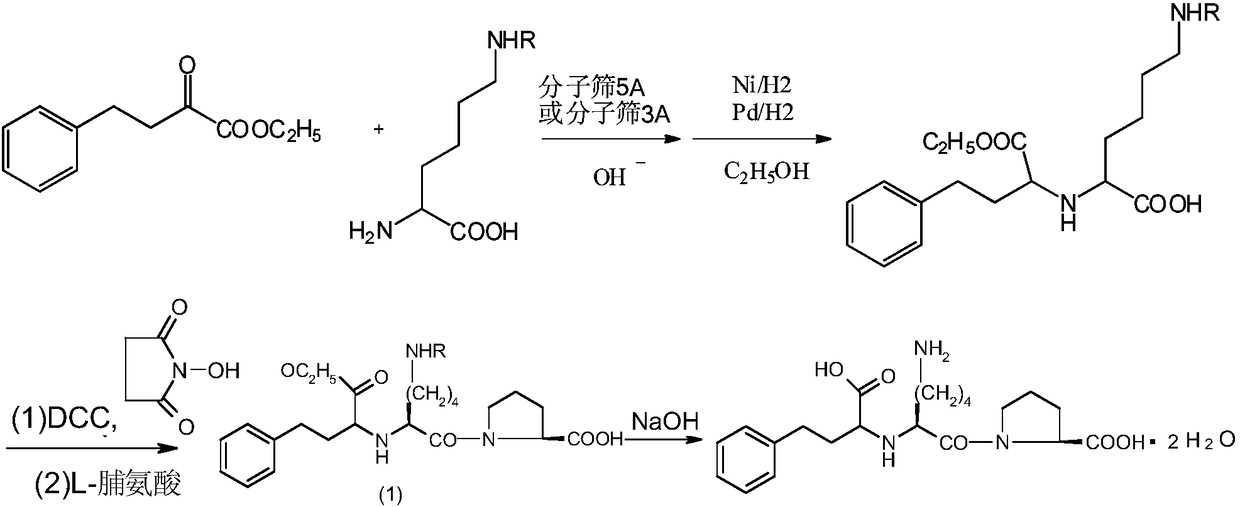
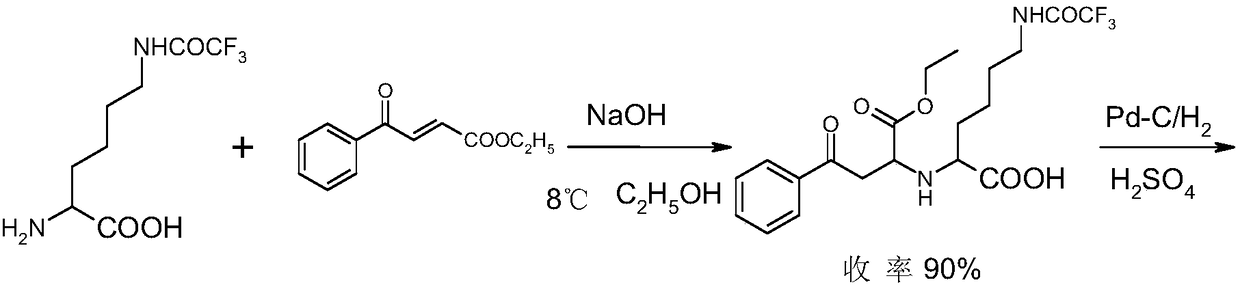
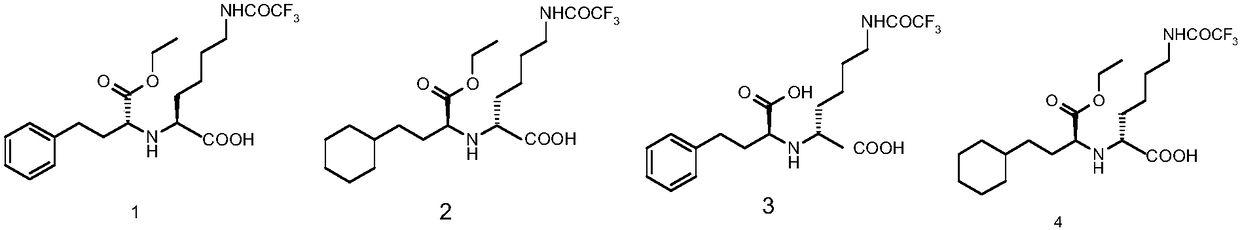
Lisinopril intermediate and purification method therefor
A purification method and pure product technology, applied in the field of lisinopril intermediates and its purification, can solve problems such as difficult product quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Lisinopril anhydride intermediate crude product preparation:
[0039] Under nitrogen protection, add 100g lisinopril hydride (formula II), 600mL dichloromethane, 58g triphosgene to the reaction vessel, heat up to 40±2°C and reflux for 20±4 hours; Concentrate under reduced pressure at 45°C to obtain 115g of the crude intermediate of lisinopril anhydride, LC-MS: 459[M+1] + .
[0040] Lisinopril anhydride intermediate purification:
[0041] Under nitrogen protection, add 400mL toluene to the crude lisinopril anhydride intermediate, adjust the temperature to 35-40°C and stir to dissolve, add 200mL of n-hexane, adjust the temperature to 35-45°C, keep stirring for 2 hours, Suction filtration at 35-45°C, wash the solid wet product with 100mL of n-hexane, and then vacuum-dry at ≤45°C for 1 hour to obtain 101g of white solid with a yield of 95.2% and a derivative HPLC purity of 98.1%, LC-MS: 459[M+1 ] + .
Embodiment 2
[0043] According to the preparation method of the crude lisinopril anhydride intermediate in Example 1, 125 g of the crude lisinopril anhydride intermediate was obtained, and 200 mL of toluene was added thereto under the protection of nitrogen, and the temperature was adjusted to 20-30° C., stirred and dissolved, and 800 mL of n-hexane was added. , adjust the temperature to 30-40°C, keep stirring for 2 hours, then suction filter at 30-40°C, wash the solid wet product with 100mL of n-hexane, and dry it in vacuum at ≤45°C for 1 hour to obtain 103g of white solid, with a yield of 97.2% , Derivative HPLC purity 97.5%, LC-MS: 459[M+1] + .
Embodiment 3
[0045] According to the preparation method of the crude lisinopril anhydride intermediate in Example 1, 108 g of the crude lisinopril anhydride intermediate was obtained, and 250 mL of toluene was added thereto under the protection of nitrogen, and the temperature was adjusted to 60-65 ° C and stirred to dissolve, and 250 mL of n-heptyl Alkanes, adjust the temperature to 25-35°C, heat and stir for 2 hours, then cool down to 10-20°C and filter with suction, wash the solid wet product with 100mL of n-heptane, and dry it in vacuum at ≤45°C for 1 hour to obtain 102g of white solid, which is collected Yield 96.2%, derivatized HPLC purity 98.3%, LC-MS: 459[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com