Method for resolving Lisinopril hydride through biocatalysis
A technology of biocatalysis and hydride, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problem of low selectivity and achieve the effects of good stereoselectivity, low environmental pollution and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of biocatalyst
[0033](1) Inoculate Acinetobacter sp.Cxzy-L119 into LB medium and culture at 30°C for 1-2 days to obtain slant cells; the concentration of the slant medium consists of: NaCl 10g / L, yeast extract powder 5.0 g / L, peptone 10g / L, pH adjusted to 7.0, sterilized at 121°C for 20min;
[0034] (2) Inoculate the slant bacteria into the seed medium, cultivate at 30°C for 24h, and obtain the seed liquid; the concentration of the seed medium consists of: NaCl 0.5g / L, MgSO 4 ·7H 2 O 1.0g / L, K 2 HPO 4 1.0g / L, NH 4 NO 3 1.0g / L, peptone 10g / L, yeast extract powder 5.0g / L, olive oil 2.0g / L, sterilized at 121°C for 20min;
[0035] (3) Inoculate the seed solution into the fermentation medium with an inoculum volume concentration of 1% to 10%, cultivate it at 30°C and 180r / min for 48h, centrifuge the fermentation solution, discard the supernatant, and obtain wet bacteria ; The concentration of the fermentation medium consists of: NaCl ...
Embodiment 2
[0037] Embodiment 2: reaction solution monitoring method
[0038] The reaction solution was washed with 5% Na 2 CO 3 The solution was adjusted to a pH of about 7.5, extracted with ethyl acetate, and the upper organic phase was retained. Take an appropriate amount of the upper organic phase, dry it with nitrogen, add the mobile phase to dissolve, mix and filter with a 0.45 μm microporous membrane, and wait for sample injection. HPLC analysis condition: mobile phase: acetonitrile: water: trifluoroacetic acid=50: 50: 0.1; Flow rate: 1mL / min; Detection ultraviolet wavelength: 210nm; Column temperature: 30 ℃; Injection volume: 10 μ L; , 4.6×250nm).
[0039] (S,S)-lisinopril hydride enantiomeric excess e.e. and substrate conversion rate are calculated according to the following formula:
[0040] Formula 1:
[0041]
[0042] Formula 2:
[0043]
[0044] where [A] SS and [A] RS Respectively, liquid chromatography records the peak area of substrate SS and RS type in the ...
Embodiment 3
[0045] Embodiment 3: lisinopril hydride small test catalytic process
[0046] 300mL catalytic reaction system: 50g / L concentration of lisinopril hydride in swirling; 30mL methanol as a co-solvent: 10g of the biocatalyst prepared in Example 1.
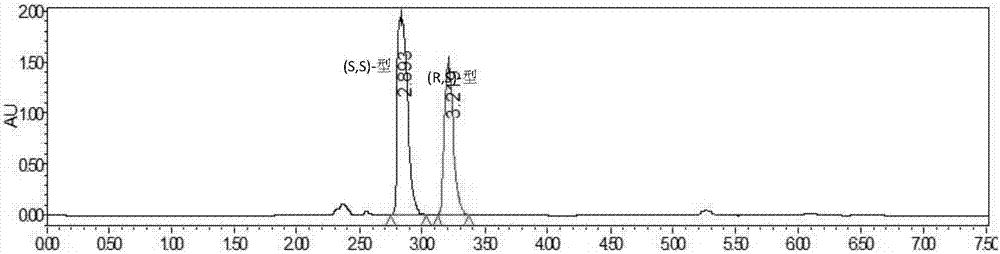
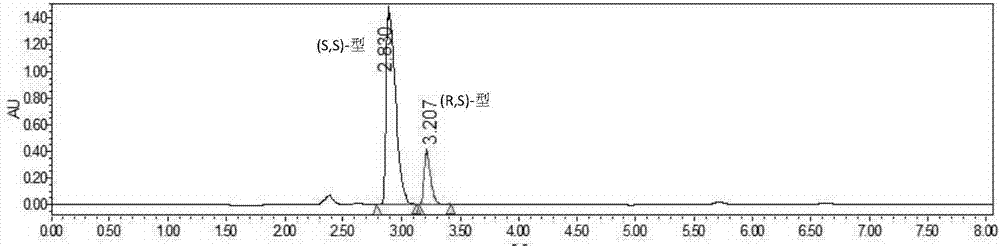
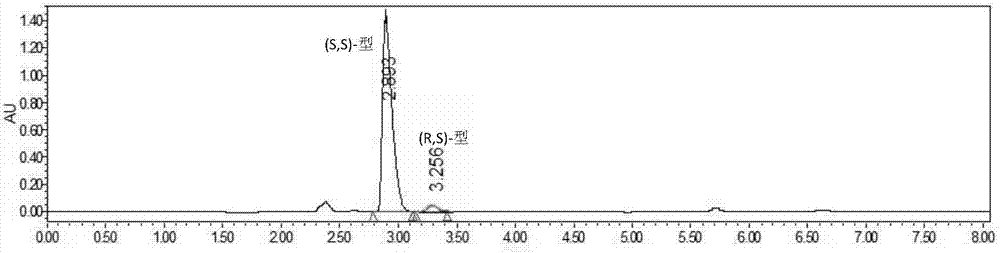
[0047] Add 15g of vortexed lisinopril hydride into a 500mL transformation bottle, add 30mL of methanol and start stirring to dissolve, then add 270mL of water, and use Na 2 CO 3 The solution (15%, V / V) adjusted the pH to around 7.2. The transformation temperature was 37° C., and 10 g of the immobilized cells prepared in Example 1 were added to start the transformation. use Na 2 CO 3 The solution (15%, V / V) maintained the pH at 7.0-7.3. Utilize HPLC to detect reaction transformation result, see table and accompanying drawing.
[0048] Table 1 Bioresolution of lisinopril hydride results
[0049] Catalytic time Conversion rate C enantiomeric excess e.e The main configuration of the remaining substrate 0h 0% 33....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com