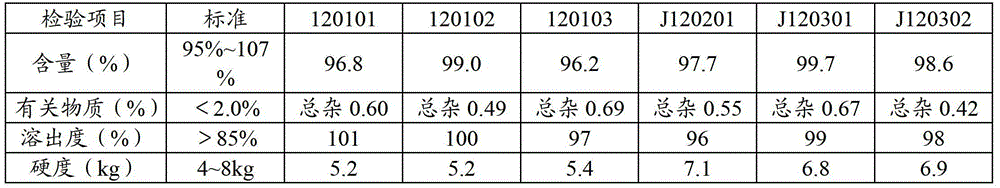
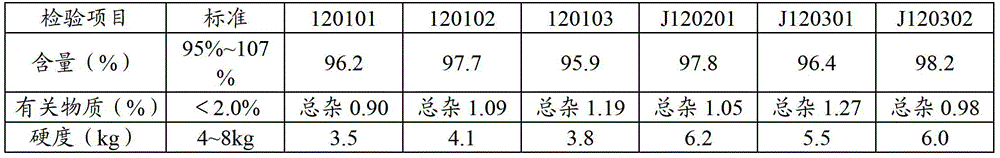
Stable lisinopril tablet and preparation method thereof
A stable and prescription technology, applied in the field of medicine, can solve problems such as difficult to control moisture, loose tablets and tablets are susceptible to moisture and water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Get lisinopril 100g, calcium hydrogen phosphate 400g, microcrystalline cellulose 450g, starch 250g, hydroxypropyl cellulose 100g, calcium sulfate 450g, carboxymethyl starch sodium 30g and hypromellose 4g, cross 30 mesh sieves;
[0048] Tablets, the tablets are pulverized with a 20-mesh straight cylinder stainless steel sieve machine;
[0049] Add 15 g of magnesium stearate, mix uniformly, and compress into tablets to obtain plain tablets.
[0050] Take PVPS63060g, 95% ethanol 940g to prepare coating solution;
[0051] Put the plain tablets in the coating pan, and start spraying liquid when the temperature of the material in the coating pan reaches 35°C, and control the temperature of the material below 40°C during the entire coating process until all the spraying is completed;
[0052] After the coating is finished, the material is completely cooled and discharged.
Embodiment 2
[0054] Get lisinopril 50g, calcium hydrogen phosphate 200g, microcrystalline cellulose 225g, starch 125g, hydroxypropyl cellulose 50g, calcium sulfate 225g, carboxymethyl starch sodium 15g and hypromellose 2g, pass through a 30-mesh sieve;
[0055] Tablets, the tablets are pulverized with a 20-mesh straight cylinder stainless steel sieve machine;
[0056] Add 7.5 g of magnesium stearate, mix well, and tablet to obtain plain tablets.
[0057] Take 30g of PVPS630 and 470g of 95% ethanol to prepare coating solution;
[0058] Put the plain tablets in the coating pan, and start spraying liquid when the temperature of the material in the coating pan reaches 35°C, and control the temperature of the material below 40°C during the entire coating process until all the spraying is completed;
[0059] After the coating is finished, the material is completely cooled and discharged.
Embodiment 3
[0061] Get lisinopril 200g, calcium hydrogen phosphate 400g, microcrystalline cellulose 350g, starch 250g, hydroxypropyl cellulose 100g, calcium sulfate 450g, carboxymethyl starch sodium 30g and hypromellose 4g, cross 30 mesh sieves;
[0062] Tablets, the tablets are pulverized with a 20-mesh straight cylinder stainless steel sieve machine;
[0063] Add 15 g of magnesium stearate, mix uniformly, and compress into tablets to obtain plain tablets.
[0064] Take PVPS63060g, 95% ethanol 940g to prepare coating solution;
[0065] Put the plain tablets in the coating pan, and start spraying liquid when the temperature of the material in the coating pan reaches 35°C, and control the temperature of the material below 40°C during the entire coating process until all the spraying is completed;
[0066] After the coating is finished, the material is completely cooled and discharged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com