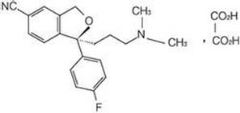
Preparation method of escitalopram oxalate drop
A technology of escitalopram oxalate and drops, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. Problems such as slow performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The technical solutions in the embodiments of the present invention will be clearly and completely described below. Obviously, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
[0034] The soda-lime glass molded pharmaceutical bottle used in the packaging complies with the regulations in "soda-lime glass molded pharmaceutical bottle" (YBB00272002-2015); 2016);
[0035] The filling volume difference index complies with the provisions of "oral solution, oral suspension, oral emulsion" and "general rule 0942 minimum filling volume inspection method" under the "general principles of preparations" in the Pharmacopoeia of the People's Republic of China.
[0036] Embodiment 1: the preparation of escitalopram oxal...
Embodiment 2
[0057] Embodiment 2: the preparation of escitalopram oxalate drops
[0058] Escitalopram Oxalate 1%
[0059] Xylitol 25.0%
[0060] Citric acid 0.4%
[0061] Sodium Citrate 1.0%
[0062] DL-malic acid 0.3%
[0063] Menthol 0.4%
[0064] Glycerin 10.0%
[0065] Propylene Glycol 30.0%
[0066] Methylparaben 0.8%
[0067] Propylparaben 0.4%
[0068] Appropriate amount of purified water
[0069] Preparation Process:
[0070] (1) Dissolution of API
[0071] The raw material drug is dissolved in an appropriate amount of purified water to obtain solution 1;
[0072] (2) Preparation of organic phase
[0073] Methylparaben, propylparaben and menthol were successively dissolved in a mixed solvent of glycerin and propylene glycol to obtain solution 2;
[0074] (3) Water phase preparation
[0075] Dissolve citric acid, sodium citrate, DL-malic acid, and xylitol in an appropriate amount of purified water to obtain solution 3;
[0076] (4) Mix solutions 1, 2, and 3 evenly, le...
Embodiment 3
[0078] Embodiment 3: the preparation of escitalopram oxalate drops
[0079] Escitalopram Oxalate 3%
[0080] Xylitol 25.0%
[0081] Citric acid 1%
[0082] Sodium Citrate 1.5%
[0083] DL-malic acid 0.6%
[0084] Menthol 0.2%
[0085] Glycerin 30.0%
[0086] Propylene Glycol 25.0%
[0087] Methylparaben 1.0%
[0088] Propylparaben 0.6%
[0089] Appropriate amount of purified water
[0090] Preparation Process:
[0091] (1) Dissolution of API
[0092] The raw material drug is dissolved in an appropriate amount of purified water to obtain solution 1;
[0093] (2) Preparation of organic phase
[0094] Methylparaben, propylparaben and menthol were successively dissolved in a mixed solvent of glycerin and propylene glycol to obtain solution 2;
[0095] (3) Water phase preparation
[0096] Dissolve citric acid, sodium citrate, DL-malic acid, and xylitol in an appropriate amount of purified water to obtain solution 3;
[0097] (4) Mix solutions 1, 2, and 3 evenly, let st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com