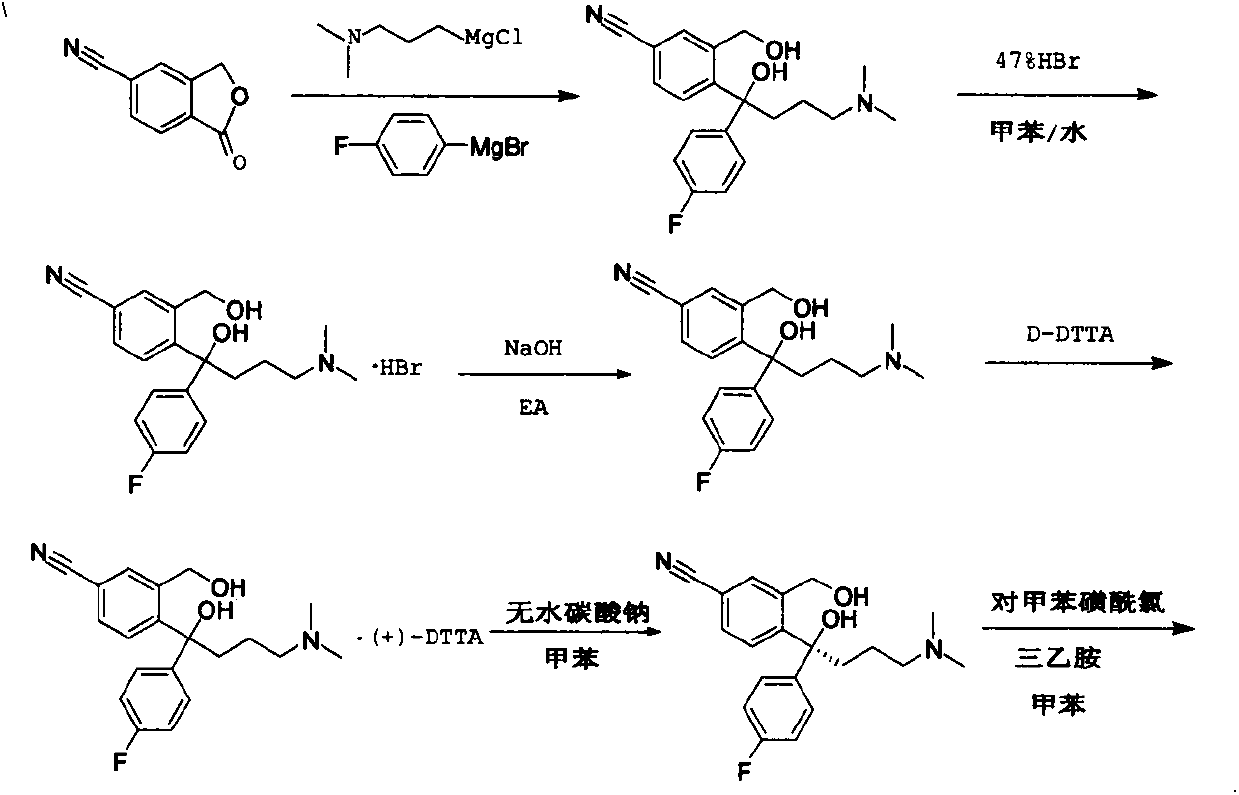
Preparation methods for impurities of escitalopram oxalate
A technology of escitalopram oxalate and citalopram amide, which is applied in the field of preparation of impurities of escitalopram oxalate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
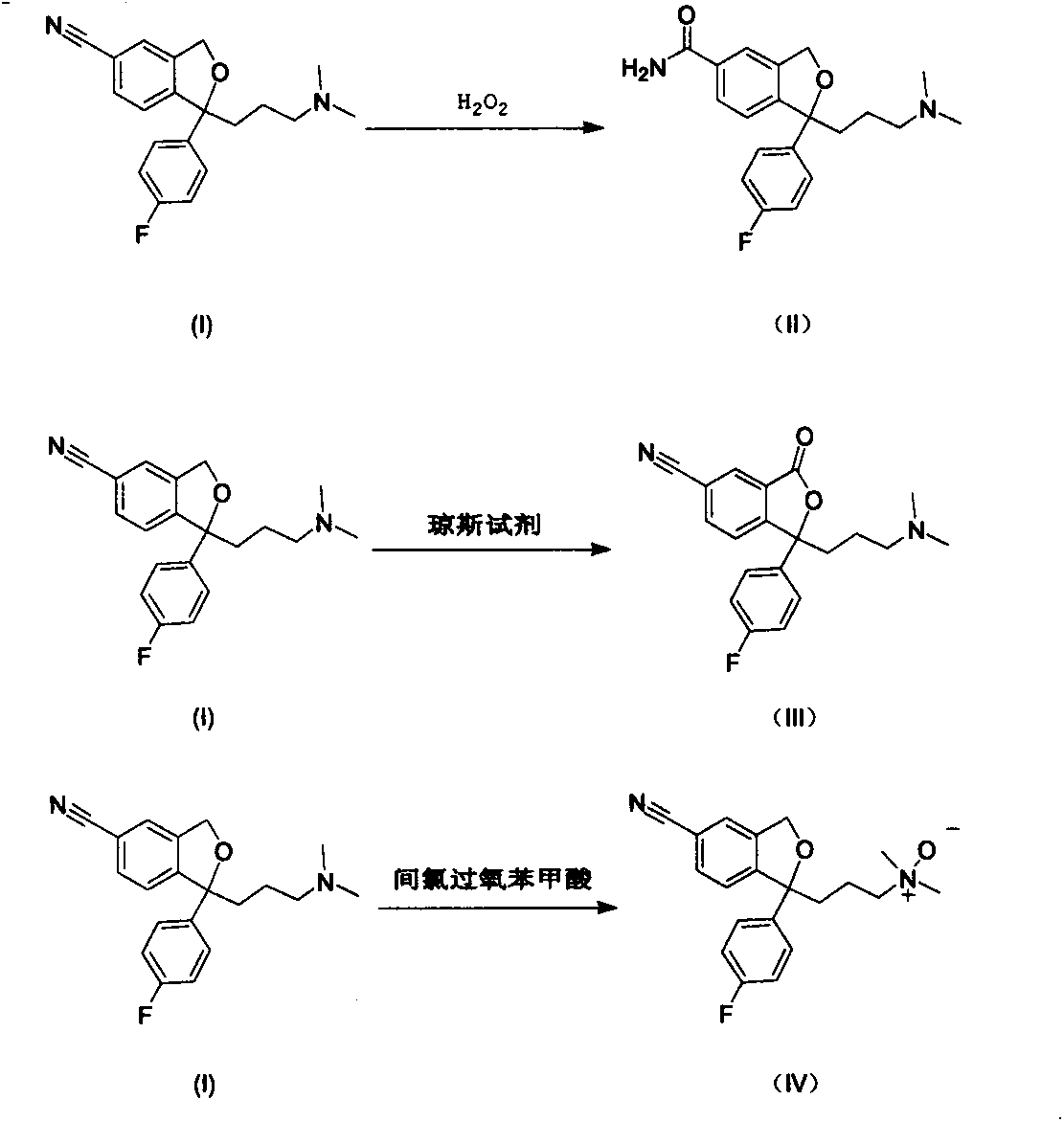
[0039] Preparation of Example 11-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carboxamide (II)
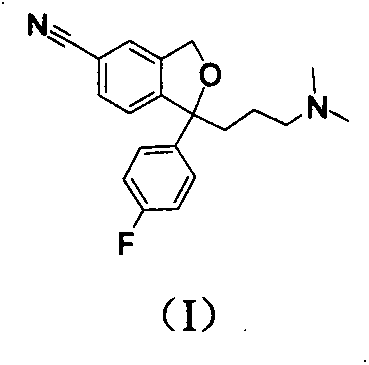
[0040] Add 2.0g of citalopram (I) into a 100ml three-necked bottle, add 80ml of methanol and stir to dissolve at 0-5°C, adjust the pH to 10 with dilute sodium hydroxide solution, add 0.6ml of 30% hydrogen peroxide dropwise , and then added 0.2ml of 30% hydrogen peroxide every 30min until the TCL tracking reaction was complete. Add an appropriate amount of saturated sodium bisulfite solution and stir until the starch potassium iodide test paper does not change color, then adjust the pH to alkaline with dilute aqueous sodium hydroxide solution, extract with n-butanol / ethyl acetate (15ml×3), combine the organic layers, and anhydrous Dry over sodium sulfate and concentrate under reduced pressure to obtain crude product (II). Silica gel column chromatography (methanol / dichloromethane=1 / 3) gave 0.9 g of pure white solid (II), with a yield of 42.6%.
[0041] I...
Embodiment 21
[0044] Example 2 Preparation of 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3-oxo-1,3-dihydroisobenzofuran-5-carbonitrile (III)
[0045] Add 8.0 g of citalopram and 80 ml of acetone into a 250 ml three-necked bottle, stir and dissolve at 0-5°C, add 30 ml of Jones reagent dropwise, stir for 30 min, and move to room temperature to react overnight. Add an appropriate amount of isopropanol, stir for 30 minutes, filter with diatomaceous earth, adjust the pH of the filtrate to 13 in an ice-water bath, extract with ethyl acetate (20ml×3), combine the organic layers, wash with saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure. The isopropanol was dissolved, and oxalic acid was added to form a salt, and the isopropanol was recrystallized to obtain 6.0 g of pure light yellow oil (III), with a yield of 75.8%.
[0046] Its structural identification data are as follows:
[0047] 1 H-NMR (DMSO-d 6 )δ: 8.45(s, 1H), 8.28(d, 1H), 8.08(d, 1H), 7.6...
Embodiment 31
[0049] Example 3 Preparation of 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisopropylfuran-5-carbonitrile-N-oxide (IV)
[0050] Add 2.34g citalopram base (I) in the 250ml three-necked bottle, dichloromethane 50ml, stirring and dissolving under ice-salt bath condition, add dropwise the dichloromethane solution 50ml that contains m-chloroperoxybenzoic acid 1.72g, dropwise Finished and continued to stir for 2h. The reaction solution was quenched with sodium bisulfite solution, concentrated under reduced pressure, added an appropriate amount of water to the residue, added dilute sodium hydroxide solution to adjust the pH to alkaline, extracted with n-butanol / ethyl acetate (20ml×3), anhydrous sulfuric acid Dry over sodium, concentrate under reduced pressure to obtain the crude product (IV), and perform silica gel column chromatography (n-butanol / ethyl acetate=1 / 3) to obtain 0.13 g of pure light yellow oil (IV), with a yield of 5.6%.
[0051] Its structural identific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com