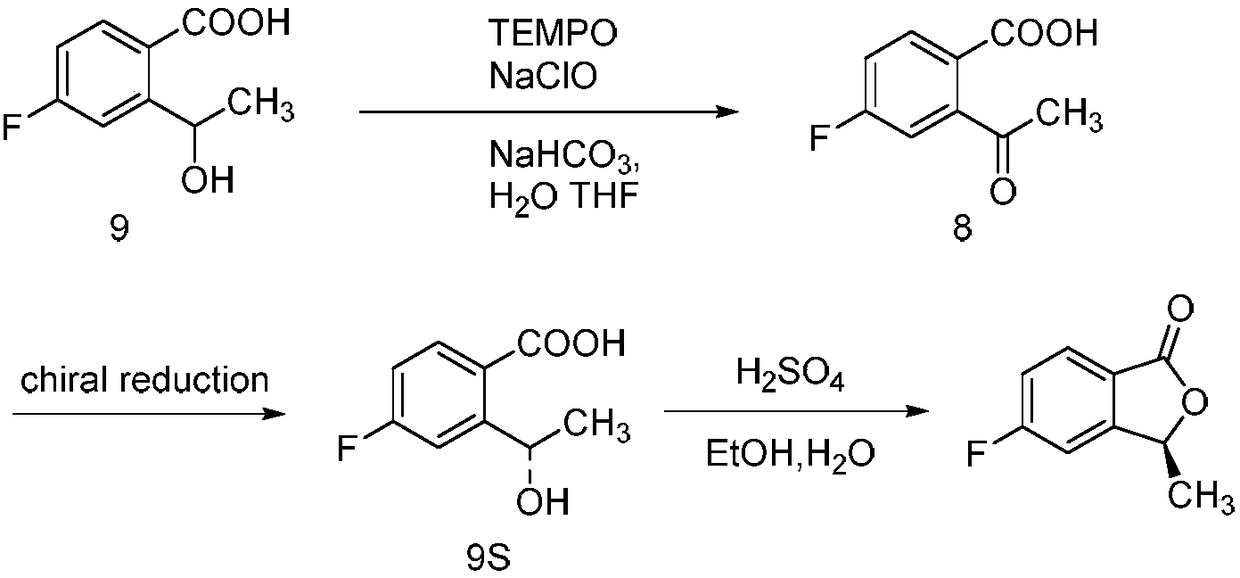
Synthesizing method of 5-fluoro-3-methyl isobenzofuran-1(3H)-ketone
A technology of methyl isobenzene and formylbenzoic acid, which is applied in the field of medicine, can solve the problems of long synthetic route, high cost, and long time-consuming, and achieve the effects of low cost, short time-consuming, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
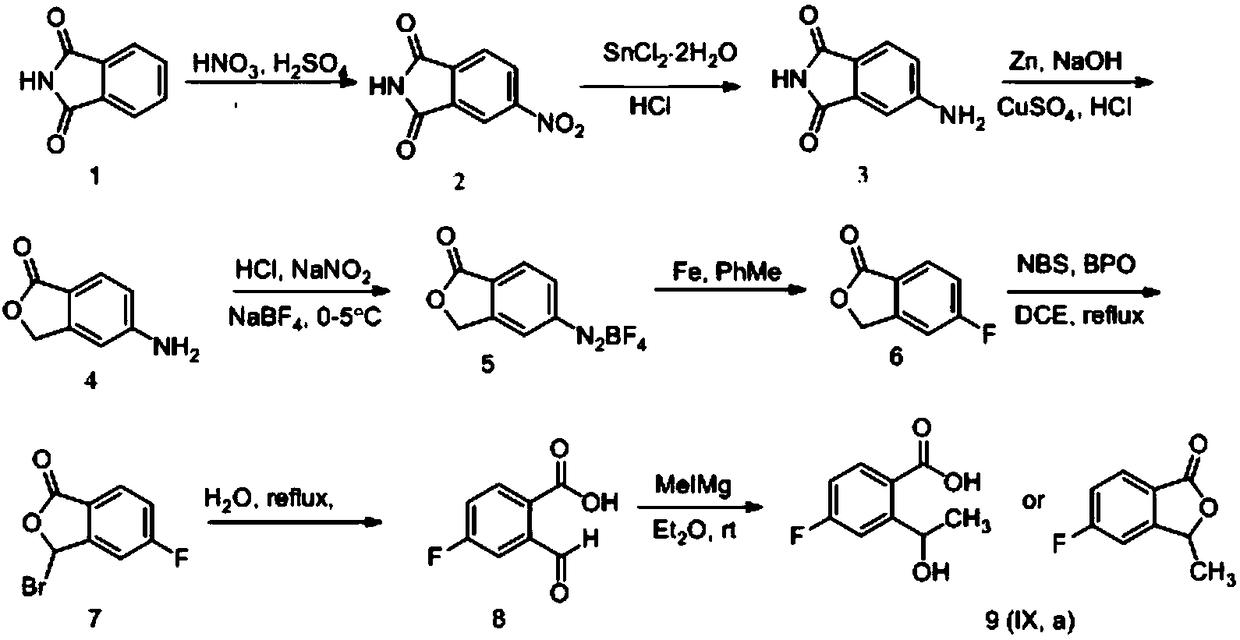
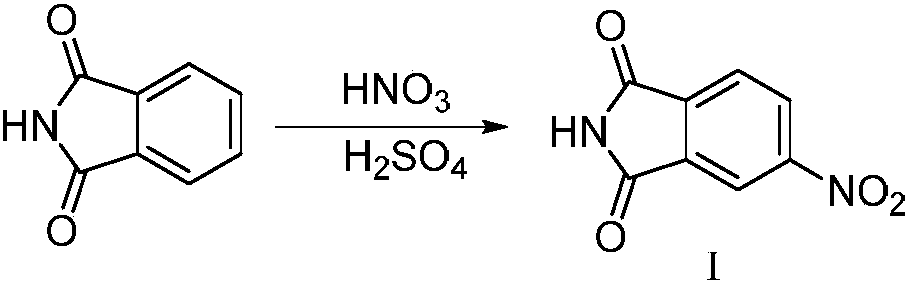
[0040] (1) Synthesis of 4-nitrophthalimide
[0041]
[0042] 1L three-neck flask, equipped with thermometer and mechanical stirring device, dissolve phthalimide with 160ml concentrated sulfuric acid, cool to below 10℃, add dropwise the prepared mixed acid (142.4ml concentrated sulfuric acid is added to 155.3ml under ice bath Concentrated nitric acid solution), the reaction is exothermic, the temperature is controlled at 15-20 ℃, the dripping is completed, stirring at room temperature for 3 hours, the reaction solution is poured into 2 times the amount of crushed ice, stirring, suction filtration, suction filtration, water washing Yellow solid, natural drying at room temperature, feed 150g, obtain white powdery solid 146.4g, yield 74.7%. ESI-MS (m / z): 193.0 [M+H]+, 385.0 [2M+H]+.
[0043] (2) Synthesis of 4-aminophthalimide
[0044]
[0045] 2L three-necked flask, equipped with thermometer and mechanical stirring device, add hydrochloric acid, add stannous chloride under stirring, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com