Pharmaceutical composition containing escitalopram oxalate and preparation method of pharmaceutical composition
A technology of escitalopram oxalate and composition, which is applied in the field of pharmaceutical composition containing escitalopram oxalate and its preparation, can solve the problems of uneven tablet content and poor material fluidity, and solve the problem of material flow poor sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0036]
[0037]
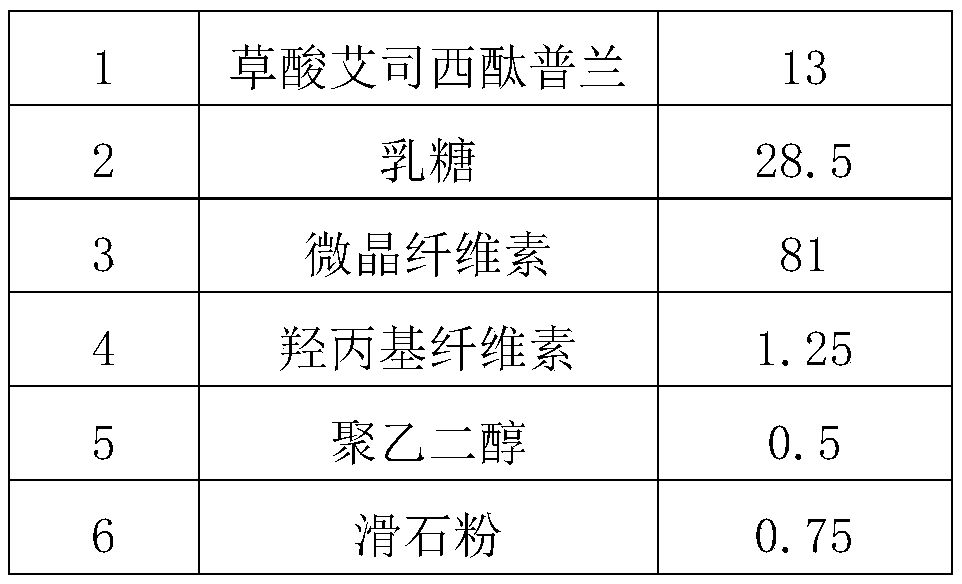
[0038] Weigh 13g of escitalopram oxalate and 41g of microcrystalline cellulose and mix them through a 80-mesh sieve to obtain mixture I; weigh 28.5g of lactose and the remaining 40g of microcrystalline cellulose and mix them through an 80-mesh sieve to obtain mixture II . Pass mixture I, mixture II and 0.75 g of talcum powder through a 60-mesh sieve, pre-mix with fluidized bed mixture for 5 minutes, mix 2.0% hydroxypropyl cellulose for fluidized bed granulation, and dry in a fluidized bed for 10 minutes. Sieve and adjust the granules to obtain granules, weigh 0.5 g of polyethylene glycol 6000 and mix for 3 minutes to make tablets.
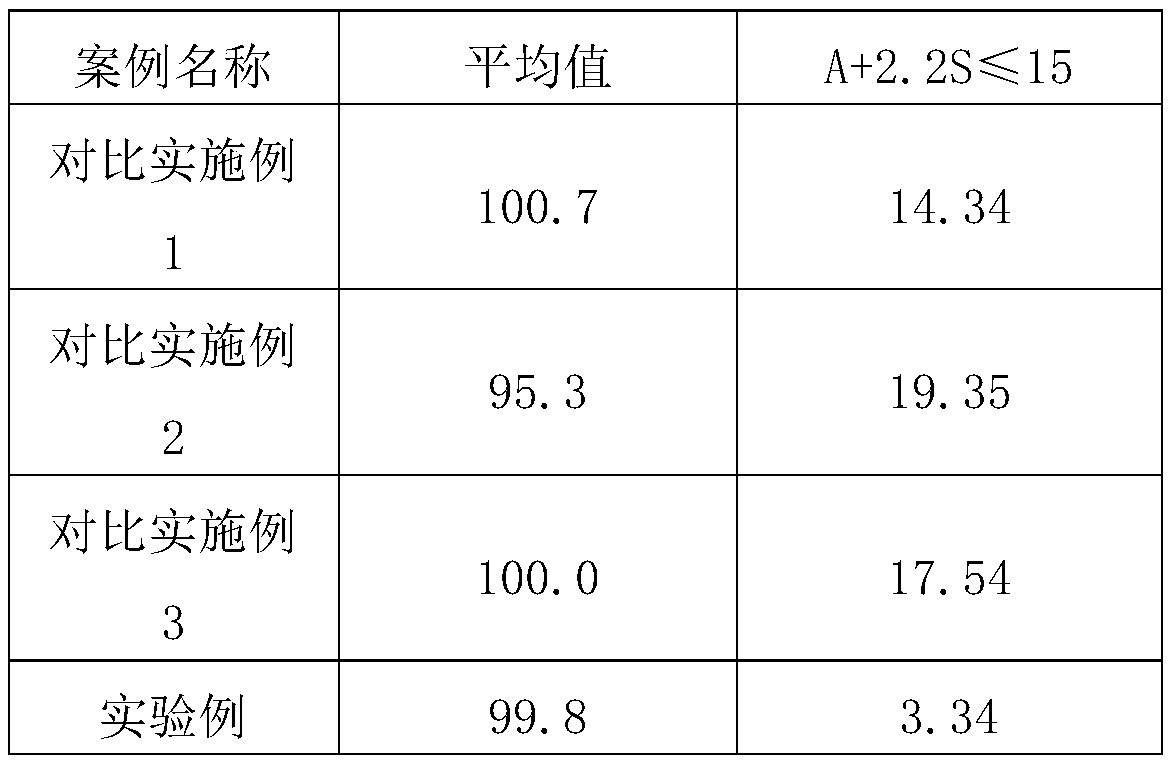
[0039] According to the content uniformity determination method (Chinese Pharmacopoeia 2015 edition general rule 0941 content uniformity inspection method), according to the pharmacopoeia standard, if A+2.2S≤15 is qualified, the results are shown in Table 1
[0040] Table 1 Test results of content uniformity
[0041]
...
Embodiment 1
[0050] serial number Material name Dosage (g) 1 Escitalopram Oxalate 25 2 lactose 50 3 microcrystalline cellulose 155 4 Hydroxypropyl Cellulose 3.0 5 polyethylene glycol 1.0 6 talcum powder 2.0
[0051] Weigh 25.0 g of escitalopram oxalate and 100 g of microcrystalline cellulose and mix them through a 80-mesh sieve to obtain mixture I; weigh 50 g of lactose and the remaining 55 g of microcrystalline cellulose and mix them through an 80-mesh sieve to obtain mixture II . Pass mixture Ⅰ, mixture Ⅱ and 2.0 g of talcum powder through a 60-mesh sieve, pre-mix the mixed material in a fluidized bed for 8 minutes, mix 1.8% hydroxypropyl cellulose for fluidized bed granulation, and dry in a fluidized bed for 12 minutes. Sieve and adjust the granules to obtain granules, weigh 1.0 g of polyethylene glycol 6000 and mix for 3 minutes to make tablets.
Embodiment 2
[0053]
[0054]
[0055] Weigh 12.5g of escitalopram oxalate and 40g of microcrystalline cellulose to pass through a 60-mesh sieve to obtain mixture I; weigh 28g of lactose and the remaining 46g of microcrystalline cellulose to pass through a 60-mesh sieve to obtain mixture II . Pass mixture I, mixture II and 1.6 g of talcum powder through a 40-mesh sieve, pre-mix with fluidized bed mixture for 5 minutes, mix 5.0% hydroxypropyl cellulose for fluidized bed granulation, and fluidized bed dry for 5 minutes. Sieve and adjust the granules to obtain granules, weigh 0.5 g of polyethylene glycol 6000 and mix for 3 minutes to make tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com