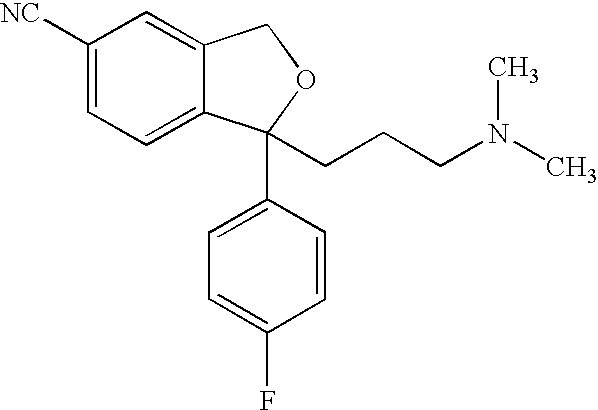
Crystalline composition containing escitalopram
a technology of crystalline composition and escitalopram, which is applied in the field of antidepressant drugs, can solve the problems of poor flow properties, poor cohesiveness or poor flow properties of active substances, and complex and expensive equipment and technical skill, and achieves the effects of improving the efficiency of escitalopram and improving the effect of escitalopram absorption and absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Scavenging of Hydroxyl Containing Impurity by Succinic Anhydride
[0082] A mixture of R— and S-Citalopram (55.5 g) containing 0.6% of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile is dissolved in dry toluene (145.0 g). Succinic anhydride (0.5 g) is added to the solution and the mixture is stirred at 45° C. (120 minutes). Water (230 ml) and aqueous ammonia (25% by weight) (3 ml) is added (pH=10.5-11.0). The phases are separated and the toluene phase is washed with water (3×120 ml). The toluene phase is evaporated and the yield is 53.0 g (95%). The product contains 0.06% of Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile.
example 2
Production Scale Crystallisation of Escitalopram Oxalate
[0083] A large number of batches of crude escitalopram oxalate have been recrystallised in production scale according to the procedure described below. The batches comprises: [0084] a) Escitalopram prepared by acidic ring-closure of the R-form of the diol precursor as described in WO03 / 000672 followed by scavenging of hydroxyl containing impurity by a production scale version of the process described in example 1 followed by separation of racemic citalopram and escitalopram as described in WO03 / 000672. These batches contain Z-4-(4-dimethylamino-1-(4-fluorophenyl)-but-1-enyl)-3-hydroxymethyl-benzonitrile, typically in the range of 0.05% (w / w) relative to escitalopram. These batches are referred to as R-diol batches. [0085] b) Escitalopram prepared by ring-closure of the S-form of the diol precursor via an activated ester under alkaline conditions as described in U.S. Pat. No. 4,943,590. These batches do not contain Z-4-(4-dimet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com