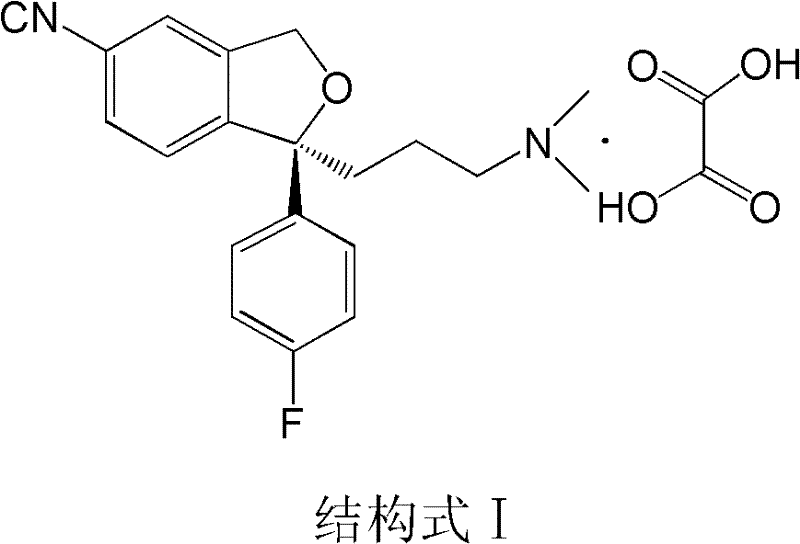
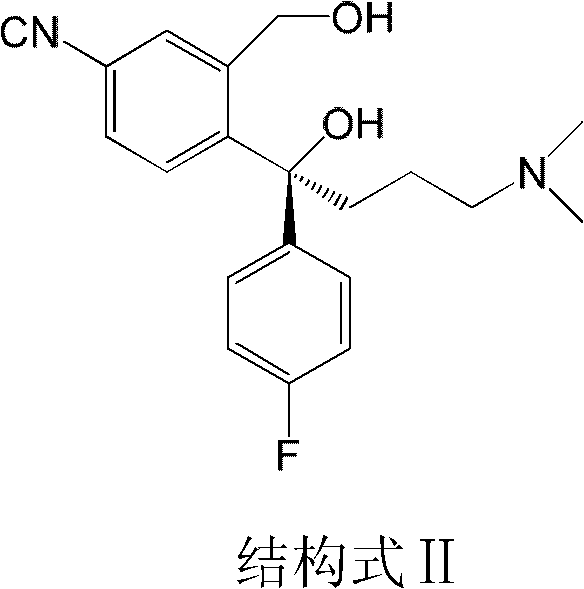
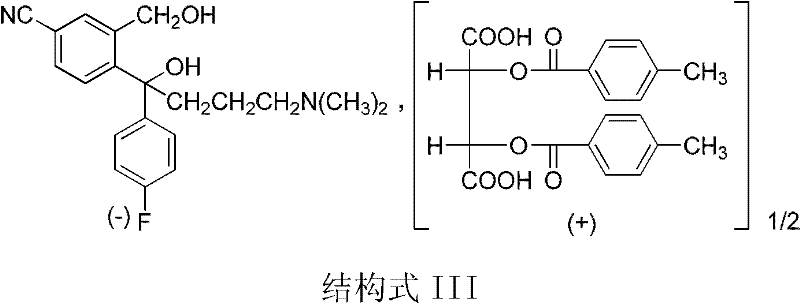
Preparation method for escitalopram oxalate
A technology of escitalopram oxalate and toluyl tartrate, which is applied in the field of medicine, can solve the problems of expensive and difficult to obtain, low yield, harsh reaction conditions, etc., and achieve easy separation and purification, high yield, The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 70.0g (0.13mol) of D-di-p-methylbenzoyl tartrate of S-type diol in 650ml of purified water, add 65ml (0.13mol) of 2mol / L sodium hydroxide solution, and control the temperature at 20±5°C Stir for 0.5 hours; after the reaction is complete, add 27.4 g (0.14 mol) of p-toluenesulfonyl chloride under control of the system temperature at 0-5° C., then add 90 ml (0.18 mol) of 2 mol / L sodium carbonate solution dropwise, and complete the dropwise addition in 0.5 hours. After the dropwise addition, the temperature was controlled at 0-5°C and the reaction was stirred for 3 hours; then, sodium hydroxide solution was added to adjust the pH value to 12-13, and the aqueous phase was extracted three times with 300ml of dichloromethane, and the organic phase was combined and washed with 200ml of purified water Once, separate the liquid, add 12.6g (0.14mol) of oxalic acid, stir for 2 hours under temperature control at 20±5°C, cool to -5~5°C, filter, and dry at 50±5°C to obtain 49....
Embodiment 2
[0028] Dissolve 70.0g (0.13mol) of D-di-p-methylbenzoyl tartrate of S-type diol in 650ml of purified water, add 65ml (0.13mol) of 2mol / L sodium hydroxide solution, and control the temperature at 20±10°C Stir for 40 minutes; after the reaction is complete, add 27.4 g (0.14 mol) of p-toluenesulfonyl chloride under control of the system temperature at 0-5 ° C, and dropwise add 90 ml (0.18 mol) of 2 mol / L sodium hydroxide solution within 40 minutes, and control the temperature Stir and react at 0-5°C for 3 hours; then add sodium hydroxide solution to adjust the pH value to 12-13, extract the aqueous phase three times with 300ml dichloromethane, combine the organic phases, wash once with 200ml purified water, separate the liquid, add 12.6g (0.14mol) of oxalic acid, stirred at 20±10°C for 2 hours, cooled to -5~5°C, filtered, and dried at 50±5°C to obtain 48.1g of white powdery solid, yield 88.8%, mp: 148.9-149.4°C.
Embodiment 3
[0030] Dissolve 70.0g (0.13mol) of D-di-p-methylbenzoyl tartrate of S-type diol in 650ml of purified water, add 65ml (0.13mol) of 2mol / L sodium hydroxide solution, and control the temperature at 20±10°C Stir at down for 0.5 hour; After the reaction is complete, add 27.4g (0.14mol) of p-toluenesulfonyl chloride under the control system temperature at 0-5°C, add 90ml (0.18mol) of 2mol / L sodium carbonate solution dropwise within 50 minutes, and control the temperature at 0 Stir and react at -5°C for 2.0 hours; then add sodium hydroxide solution to adjust the pH to 12-13, extract the aqueous phase three times with 300ml dichloromethane, combine the organic phases, wash once with 200ml purified water, separate the liquids, and add oxalic acid 12.6g (0.14mol), stirred at 20±10°C for 2 hours, cooled to -5~5°C, filtered, and dried at 50±5°C to obtain 48.5g of white powdery solid, yield 89.5%, mp: 149.9 -150.4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com