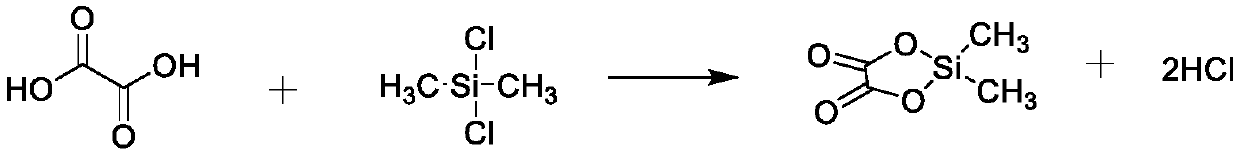Patents
Literature
466 results about "Cacodylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
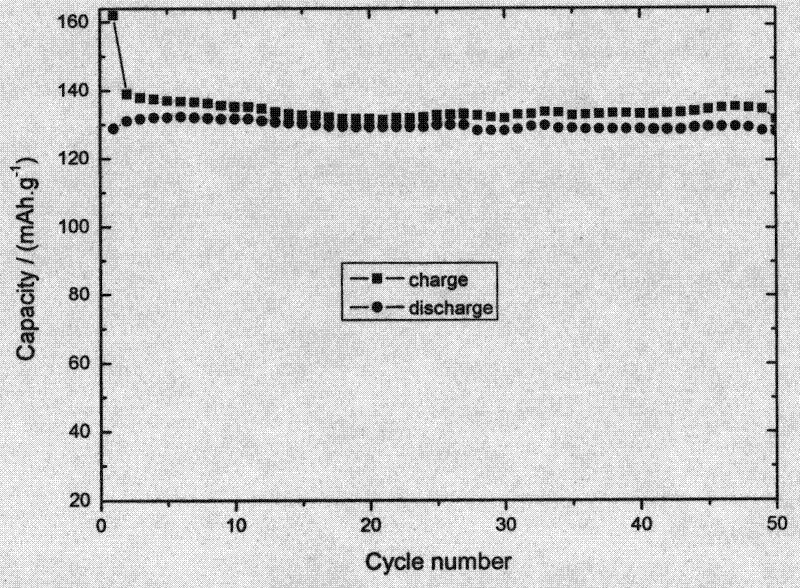
Cacodylic acid is the organoarsenic compound with the formula (CH₃)₂AsO₂H. With the formula R₂As(O)OH, it is the simplest of the organoarsinic acids. It is a colorless solid that is soluble in water. Neutralization of cacodylic acid with base gives cacodylate salts, e.g. sodium cacodylate. They are potent herbicides. Cacodylic acid/sodium cacodylate is a buffering agent in the preparation and fixation of biological samples for electron microscopy.
Solid supported noble metal catalyst and its preparing method
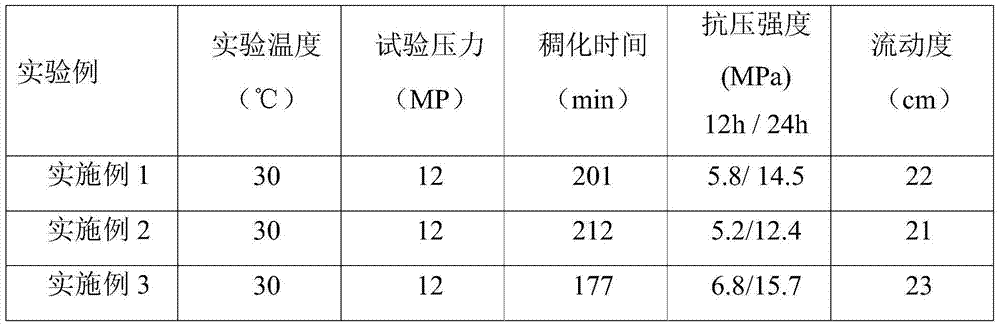
InactiveCN1425499ASmall particle sizeIncrease profitCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsOXALIC ACID DIHYDRATEAlkaline earth metal
The supported noble metal catalyst has any one of Ru, Rh, Pd, Os, Ir, Pt, Au, Ag, Cu and Ni as the active component; and any one of active carbon, graphite, carbon nanotube, Al2O3, SiO2, molecular sieve, MgO and TiO2 as the carrier. Its active component accounts for 0.1-90 % and metal particle size of 0.5-6 nm. The preparation process includes dispersing the active component, hydroxide or carbonate of alkali metal or alkali earth metal and carrier in solvent separately; mixing in certain proportion, heating the solution, adding nitric acid, sulfuric acid, hydrochloric acid, oxalic acid or acetic acid as precipitation promoter; and stoving. The catalyst is used as catalyst for fuel cell and makes the fuel cell possess high performance.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of structured flue gas denitration catalyst, prepared catalyst and application of catalyst
ActiveCN102166514AReduce oxidation rateHigh mechanical strengthDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsTungstateNitric oxide
The invention relates to a preparation method of structured flue gas denitration catalyst, a prepared catalyst and application of the catalyst. The method comprises the following steps of: pre-mixing TiO2 powder and ammonium meta-tungstate-containing oxalic acid solution to prepare WO3-containing TiO2 powder; mixing the prepared TiO2 powder, an inorganic binder, a plasticizer, glass fibers and sesbania powder in a special mass ratio; adding oxalic acid solution of ammonium metavanadate and aqueous solution of cerous nitrate; mixing the materials; adding ammonia water to adjust the pH value tobetween 9 and 10; ageing the mixture for 5 to 10 days; coating the aged mud material onto a stainless steel net; after rolling the aged mud, forming, and drying and roasting the prepared plate type structural V2O5 / TiO2-based denitration catalyst. The plate type structural V2O5 / TiO2-based denitration catalyst has high mechanical strength and catalytic activity and low SO2 oxidation rate, so the catalyst can be widely applied to the removal of nitric oxide in coal / oil / gas boilers and nitric acid industry.
Owner:CHINA DATANG TECH & ENG
Trivalent-chromium blue-white passivator for zinc-plated permanent magnetic material and passivation method thereof
InactiveCN102041497AAvoid pollutionAvoid damageMetallic material coating processesRare-earth elementNickel salt
The invention provides a trivalent-chromium blue-white passivator for a zinc-plated permanent magnetic material and a passivation method thereof. The trivalent-chromium blue-white passivator contains a main film-forming agent, an auxiliary film-forming agent, a stabilizer, an oxidant and a surfactant, wherein the main film-forming agent is a soluble salt of trivalent chromium, the stabilizer is selected from at least one of fluoride, citric acid and oxalic acid, the auxiliary film-forming agent is selected from at least one of soluble cobalt salt, soluble nickel salt or soluble salts of rare-earth elements, the oxidant is nitrate ions or hydrogen peroxide, and the surfactant is NPE (Nonylphenol Polyoxyethylene Ether) or sodium dodecyl benzene sulfonate. The trivalent-chromium blue-white passivator provided by the invention has a low cost, does not pollute the environment, and also does not cause damage to the health of operators.
Owner:BEIJING ZHONG KE SAN HUAN HI TECH +1
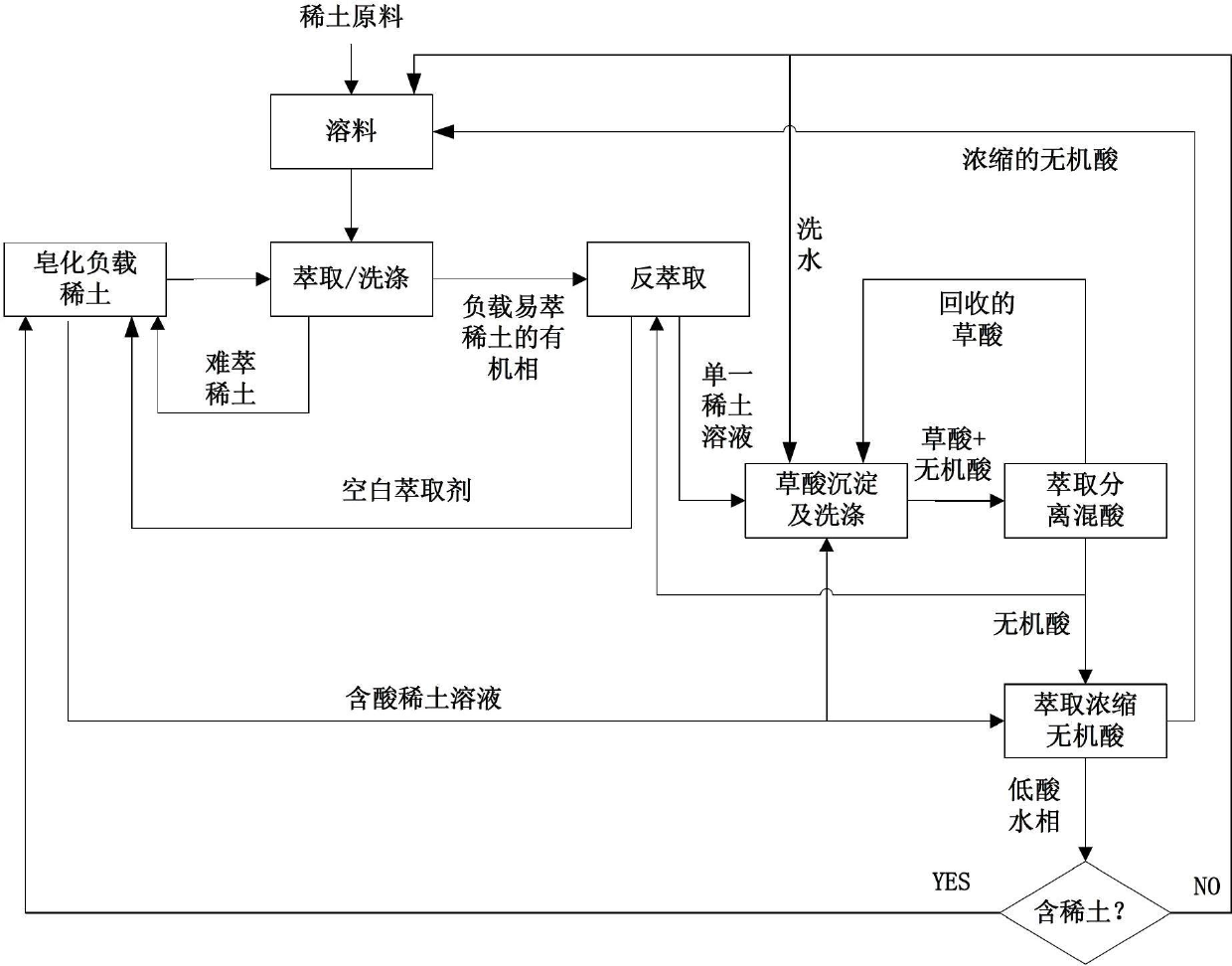
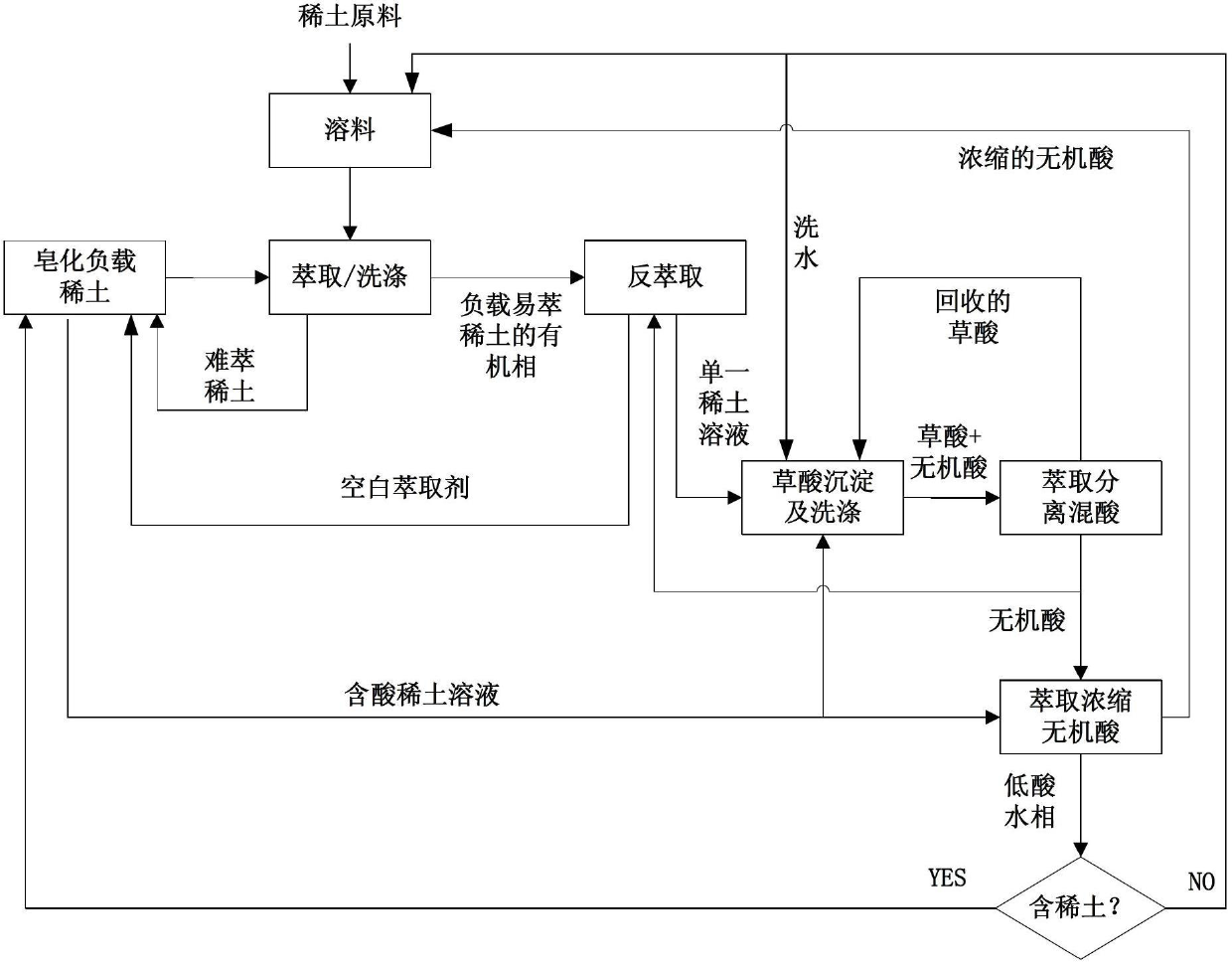
Rare earth separation method with material linkage cyclic utilization function
ActiveCN102676853ALow costSolve the problem of low extraction capacityProcess efficiency improvementOxalateCacodylic acid
The invention relates to a rare earth separation method with the material linkage cyclic utilization function. The rare earth separation method comprises the following steps: the organic phase of loaded rare earth prepared by extractant A and rare earth soap stock through mixing is used for the follow-up linkage extraction separation, and inorganic acid in the residual water phase is extracted and concentrated by extractant C and is then reused for material dissolving or recovering rare earth with oxalic acid precipitated therein; purified rare earth solution is subjected to extraction separation, the rare earth is precipitated through oxalic acid, sediment mother solution containing oxalic acid and inorganic acid is mixed with extractant B, the oxalic acid is extracted to be reused for precipitating rare earth, and the residual inorganic acid is directly used for washing and back extraction processes or is used for material dissolving after being concentrated by the extractant C. With the rare earth separation method provided by the invention, intermediate materials generated in the rare earth separation process can be recycled in process sections in a linkage manner, the alkali saponification process is avoided, and the material dissolving, washing and back extraction processes can be finished only by the recycled inorganic acid, so that the rare earth separation and purification process disclosed by the invention does not consume alkaline and inorganic acid, the cost is low, and the rare earth separation method is green and environmental-friendly.
Owner:CHINA MINMETALS BEIJING RES INST OF RE
Pesticide compositions containing oxalic acid
InactiveUS6992045B2Easy to controlIncreased toxicityBiocideDead animal preservationOxalateHydroxyproline
Pesticidal concentrate and spray compositions are described which exhibit enhanced efficacy due to the addition thereto of a compound which increases cell membrane permeability, suppresses oxidative burst, or increases expression of hydroxyproline-rich glycoproteins.
Owner:MONSANTO TECH LLC
Method for preparing vanadium oxide
InactiveCN103146930AAchieve recyclingReduce consumptionProcess efficiency improvementHigh phosphateSlag
The invention provides a method for preparing vanadium oxide, which comprises the following steps of: mixing vanadium slag with calcium oxide or limestone to form a mixed material; roasting the mixed material to obtain calcified clinker; leaching the calcified clinker by using C2O4<2-> and 35-70 g / L of oxalate solution at 80-95 DEG C; after leaching, carrying out solid-liquid separation to obtain vanadium-containing leachate and residues; removing silicon from the vanadium-containing leachate, so that the silicon concentration in the vanadium-containing leachate is less than 0.1 g / L, then adding ammonium oxalate into the vanadium-containing leachate, adjusting the mol ratio of NH4<+> to TV to 2-3.5, precipitating ammonium metavanadate, and filtering to obtain ammonium metavanadate and vanadium precipitation wastewater; and oxidizing, roasting and deaminizing ammonium metavanadate to prepare vanadium pentoxide or reducing to prepare vanadium trioxide. According to the invention, on the premise of satisfying environment-friendly requirements, preparation of vanadium oxide from ordinary vanadium slag and high-calcium and high-phosphate vanadium slag is realized; furthermore, consumption of reagents can also be reduced; and the production cost is reduced.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP
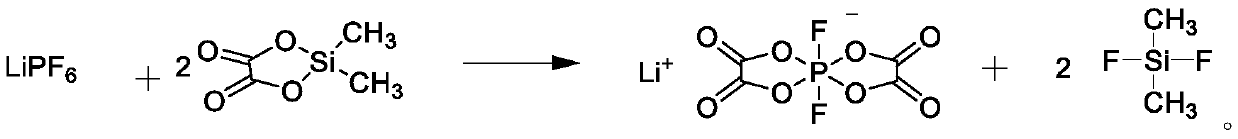
Preparation method of lithium difluorobisoxalate phosphate solution
The invention provides a preparation method of a lithium difluorobisoxalate phosphate solution. The preparation method of the lithium difluorobisoxalate phosphate solution comprises the following steps that (1) dichlorodimethylsilane and oxalic acid are reacted in the presence of a non-aqueous solvent to generate dimethyl silicon-based oxalate; and (2) lithium hexafluorophosphate is added into thereaction solution in the step (1), and reacting is carried out to obtain the lithium difluorobisoxalate phosphate solution. The preparation method is simple and practical, and industrial production can be achieved; the prepared lithium difluorobisoxalate phosphate solution can be directly used as an additive of a non-aqueous electrolyte battery; and the prepared lithium difluorobisoxalate phosphate solution has few chlorine compounds and free acids, wherein the chlorine compounds in terms of chlorine concentration are less than 5 mass ppm, and the free acids in terms of acid concentration converted from hydrofluoric acid are less than 200 mass ppm.
Owner:JIANGSU GUOTAI SUPER POWER NEW MATERIALS
Acid pickling and waste acid treatment process for improving purity of powdery quartz sand
The invention provides an acid pickling and waste acid treatment process for improving purity of powdery quartz sand. The process includes the steps: putting the powdery quartz sand in an acid pickling purification device, and using an acid pump for pumping acid pickling solution into the acid pickling purification device, wherein the acid pickling solution is composed of hydrofluoric acid, fluorosilicic acid, oxalic acid and water or composed of fluorosilicic acid, oxalic acid and water; removing the acid pickling solution after acid pickling, and collecting; delivering the powdery quartz sand to a washing device by clear water after acid pickling, washing to neutral, and dewatering to obtain a finished product of powdery quartz sand; transferring waste acid into a neutralization tank for neutralization, delivering into a sedimentation tank by the pump, and allowing calcium fluosilicate and calcium oxalate to precipitate; delivering the clear water into a regulation tank after precipitation to realize pH adjustment for precipitation of iron ions; using oxalic acid or fluorosilicic acid to adjust pH, precipitating calcium fluosilicate and calcium oxalate again, filtering to obtain clear water, and delivering the clear water to a workshop for recycling. The acid pickling and waste acid treatment process for improving purity of the powdery quartz sand has the advantages of remarkable purity improvement and impurity reduction and simplicity and convenience in subsequent treatment.
Owner:HUANGGANG NORMAL UNIV +1
Method for synthesizing cathode material LiNi0.5Mn1.5O4 for 5V lithium ion batteries
The invention discloses a method for synthesizing cathode material LiNi0.5Mn1.5O4 for 5V lithium ion batteries. Manganese salt, nickel salt and lithium compound are used as main material, oxalic acid is used as precipitator, and aqueous ammonia is used as complexing agent; the manganese salt and the nickel salt are proportionally mixed and then dissolved in water, so that manganese salt-nickel salt mixture is produced, and a moderate amount of oxalic acid is dissolved in water, so that oxalic acid solution is produced; the manganese salt-nickel salt mixture is first blended and reacts with the aqueous ammonia to form manganese and nickel ammonia complex ions, and then is blended and reacts with the oxalic acid solution to form manganese oxalate containing nickel; the manganese oxalate containing nickel and the mother solution are directly dried, so that manganese oxalate powder containing nickel is obtained, and the manganese oxalate powder containing nickel is then roasted under the temperature of 400 DEG C to 650 DEG C for three to fifteen hours, so that manganese oxide containing nickel is obtained; and the manganese oxide containing nickel and the compound of lithium are uniformly mixed and ground, and are then roasted under the temperature of 700 DEG C to 950 DEG C for eight to thirty hours. The invention has the advantage of simple technique and easy control, and the synthesized cathode material LiNi0.5Mn1.5O4 has good electrochemical properties.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Detergent for cleaning ceramic filtering plate
InactiveCN1978616AGood removal effectPromote decompositionInorganic non-surface-active detergent compositionsFilter regenerationPhosphateCleansing Agents
This invention relates to a washing agent used in rinsing ceramic strainer plate. According to the weight percentage, it contains the following materials: nitric acid 0.1~5%; hydrochloric acid 0.1~5%; hydrofluoric acid 0.2~3%; oxalic acid 0.1~5%. By the combined action of nitric acid, hydrochloric acid and hydrofluoric acid, it can deal with the mixed dirt such as carbonate, silicate, sulphate, phosphate, sulphide and hydroxid, and so on. The washing effectiveness is good and the scope of application is extensive. Otherwise, it can enhance the operating life of strainer plate and can extend the serviceable range of ceramic filtering machine.
Owner:ANHUI TONGGUAN MACHINERY
Method for cleaning immersed ultrafiltration membrane in wastewater reuse
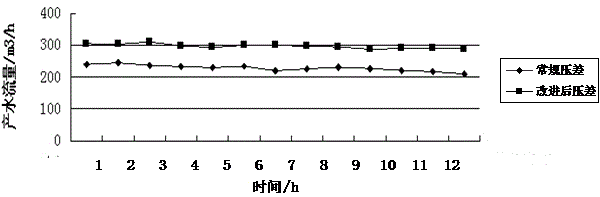
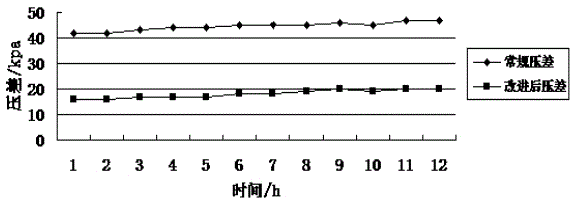
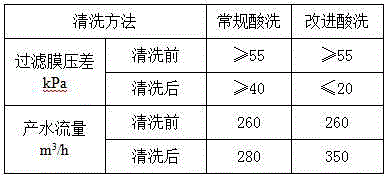
ActiveCN103949163AGood water flowGood operating differential pressureSemi-permeable membranesOXALIC ACID DIHYDRATEUltrafiltration
The invention discloses a method for cleaning an immersed ultrafiltration membrane in wastewater reuse. The method comprises the following step of chemical cleaning. The method is characterized in that the step of chemical cleaning comprises the following substeps of alkali cleaning, acid cleaning or combined alkali cleaning and acid cleaning, wherein each of the alkali cleaning method and the acid cleaning method is of a method combining the circulatory cleaning with the immersed cleaning, and an alkali cleaning agent comprises sodium hypochlorite, sodium dodecyl benzene sulfonate and sodium tripolyphosphate; an acid cleaning agent comprises citric acid, hydrochloric acid and oxalic acid. The method has the advantages that sodium dodecyl benzene sulfonate and sodium tripolyphosphate are added in the process of alkali cleaning by sodium hypochlorite, so that the cleaning effect of the method on ultrafiltration organic matters and oil pollutants is obviously better than that of a normal alkali cleaning method, the polluting and plugging degree of the cleaned ultrafiltration membrane can be reduced, and stability in operation can be realized; the acid cleaning is adopted as required after alkali cleaning, and the oxalic acid agent is added in the process of acid cleaning, so that the ultrafiltration membrane can be thoroughly cleaned, and the produced water flow and the running differential pressure of the ultrafiltration membrane can be recovered to be the optimal effect.
Owner:SHANXI TAIGANG STAINLESS STEEL CO LTD
Method for recovering organic acid and cobalt-manganese metal in terephthalic acid oxidized residues
ActiveCN102503813AOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingMANGANESE ACETATEBenzoic acid
The invention belongs to the technology for recovering organic acid and catalyst from terephthalic acid oxidized residues, which includes the steps of firstly, discharging high-temperature oxidized residues from a pure terephthalic acid (PTA for short) production device, controlling the solid content within the range from 20% to 45% and implementing primary filtering separation within the temperature range from 55 DEG C to 90 DEG C, and directly vending separated solid for manufacturing resin or paint or returning the separated solid to an oxidation reactor for use, wherein primary filtrate is treated according to the process: (I) adding oxalic acid to obtain cobalt-manganese oxalate precipitation, obtaining cobalt-manganese oxalate by means of filtering separation, further cooling the filtrate by means of filtering separation, utilizing the separated solid for extracting benzoic acid and delivering the filtrate to a waste water treating device; or (II) directly cooling the primary filtrate for secondary filtering separation, utilizing the separated solid for extracting benzoic acid, adding oxalic acid into secondary filtrate to obtain cobalt-manganese oxalate precipitation, obtaining cobalt-manganese oxalate by means of filtering separation and delivering the filtrate to a waste water treating device; and secondly, carrying out reaction of the cobalt-manganese oxalate obtained from (I) or (II) with oxidant such as hydrogen peroxide, peroxyacetic acid, bromine, manganate, permanganic acid, manganese dioxide or / and hydrobromic acid, utilizing cobalt acetate aqueous liquor, cobalt bromide aqueous liquor, manganese acetate aqueous liquor, manganese bromide aqueous liquor, acetic acid or / and pure water as dissolvent, then carrying out reaction of the cobalt-manganese oxalate with metallic cobalt, metallic manganese or / and hydrobromic acid after the cobalt-manganese oxalate is completely dissolved, and obtaining homogeneous phase liquor containing cobalt-manganese bromide ions by means of purification and filtration, wherein the mixed liquor can be directly mixed with cobalt acetate, manganese acetate, cobalt bromide, manganese bromide, acetic acid or water to serve as oxidation catalyst for the terephthalic acid.
Owner:浙江上虞利星化工有限公司
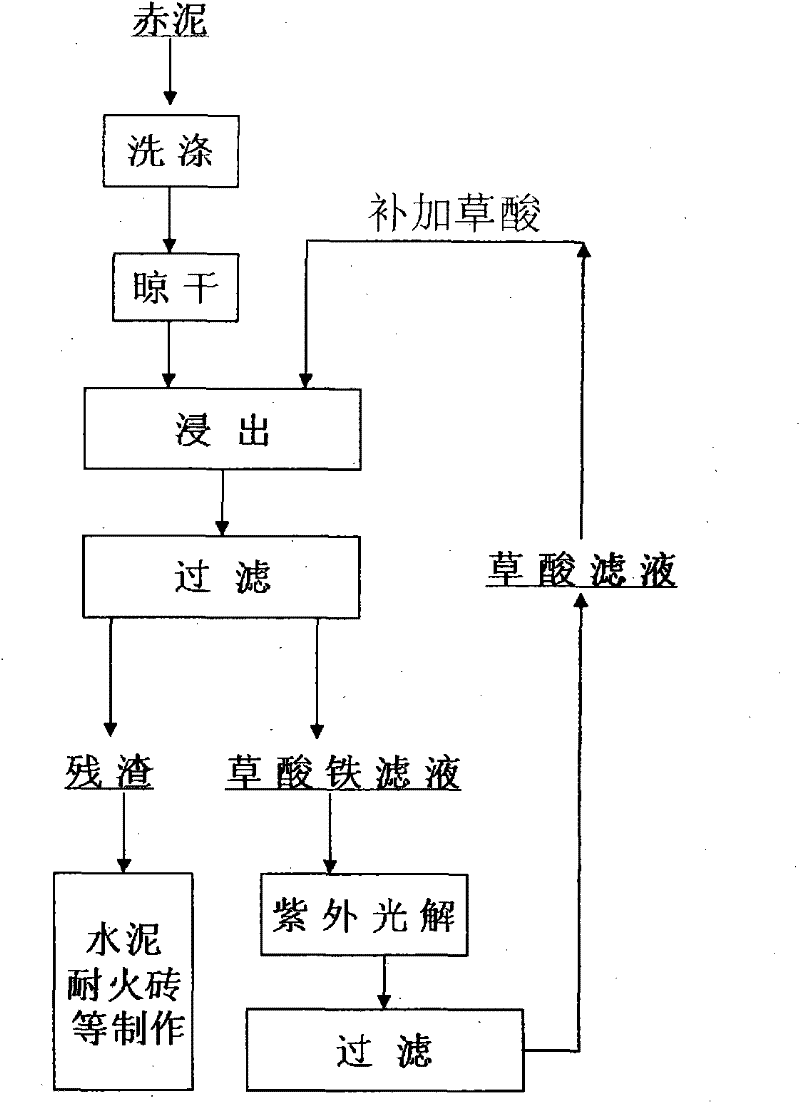
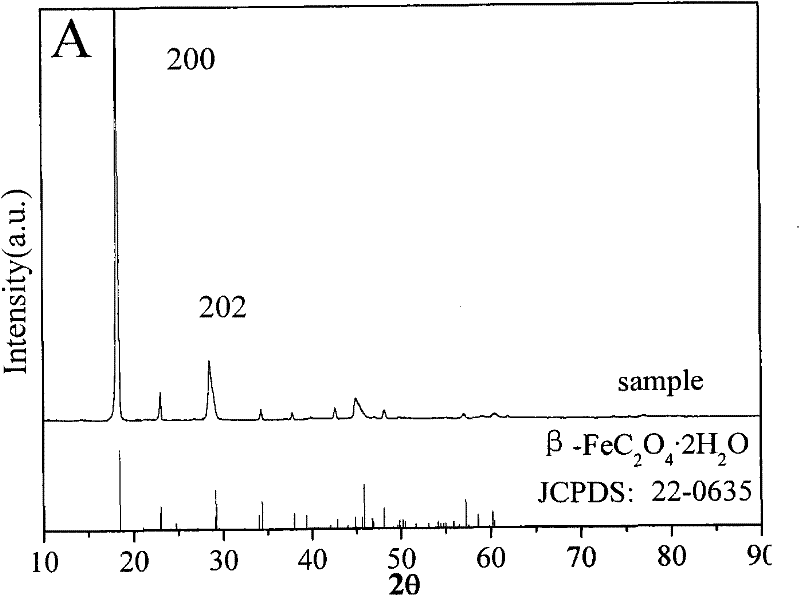
Method for recovering ferric oxide from red mud by leaching-photocatalysis by oxalic acid
A method for treating red mud by utilizing oxalic acid solution comprises the steps of taking 0.5-1mol / L oxalic acid solution as a leaching agent, mixing air-dried red mud with the oxalic acid solution according to a proportion of 80-120g / L, stirring at 60-90 DEG C for 0.5-4h, and conducting solid-liquid separation to obtain the treated red mud and ferrum-containing oxalic acid solution. The ferric oxide content in the treated red mud is smaller than 1 percent, so that the red mud is applicable to manufacturing of refractory bricks, cement and the like. The ferrum-containing oxalic acid solution is subjected to photochemical reaction under irradiation of ultraviolet light or sunshine, thus being capable of forming ferrous oxalate sedimentation with better crystal. Ferric oxide content of filtrate after being separated from ferrous oxalate sedimentation is smaller than 1g / L, and the filtrate solution is continuously used for treating the red mud as supplement liquid of the leaching agent after being added with proper oxalic acid or being directly used.
Owner:BEIJING UNIV OF CHEM TECH
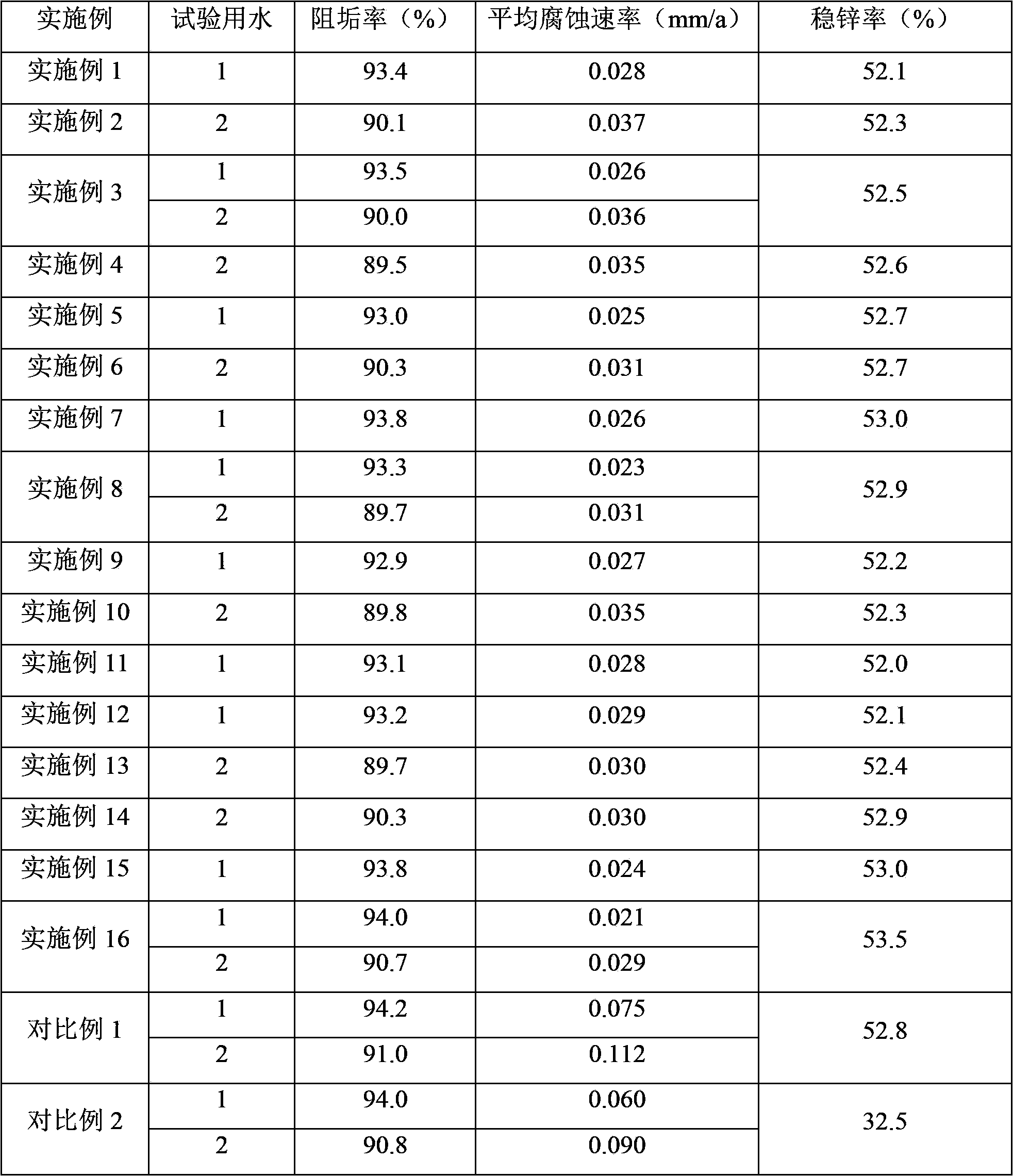
Composite corrosion and scale inhibitor, and its application in circulating cooling water treatment
ActiveCN103787516AGood anti-scaling effectGood synergistic corrosion inhibition effectScale removal and water softeningCarboxylic acidCopolymer
The invention discloses a composite corrosion and scale inhibitor, and its application in circulating cooling water treatment. The composite corrosion and scale inhibitor contains oxalic acid and / or oxalate, a sulfonic group-containing copolymer, a scale inhibition additive and a zinc salt, the scale inhibition additive is at least one of organic phosphonic acid, phosphorus-free polycarboxylic acid and phosphorus-free polyacid anhydride, and a weight ratio of oxalic acid and / or oxalate to the sulfonic group-containing copolymer to the scale inhibition additive to the zinc salt by zinc ion is 10-30:2.5-5.5:2-12:1. The composite corrosion and scale inhibitor contains no phosphorus or has a low concentration of total phosphorus, is environmentally-friendly, and is especially suitable for circulating water with the sum of the total alkalinity and the total hardness of above 100mg. The above zinc salt formula containing oxalic acid and / or oxalate realize excellent scale and corrosion inhibition effects.
Owner:CHINA PETROLEUM & CHEM CORP +1
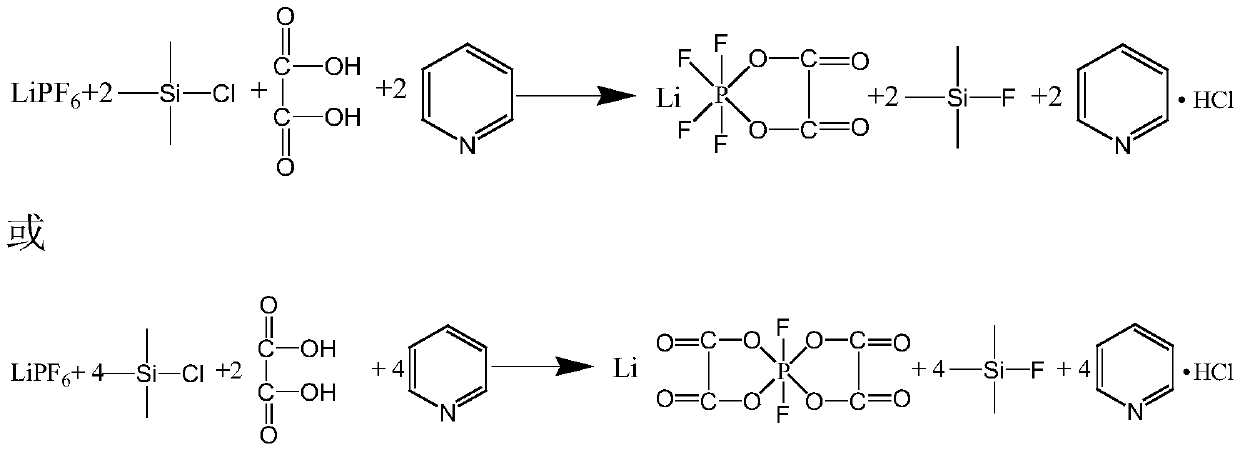
Preparation method of lithium tetrafluorooxalatophosphate and lithium difluorodioxalatophosphate
The invention relates to a preparation method of lithium tetrafluorooxalatophosphate and lithium difluorodioxalatophosphate. The method comprises the following steps: preparation of a titration solution: dissolving lithium hexafluorophosphate in an organic solvent, and adding trimethylchlorosilane into the solution; preparation of a base solution: dissolving oxalic acid into an organic solvent, preparing an organic alkali solution in the same way, adding the organic alkali into the oxalic acid solution, and stirring the solution; slowly dropwise adding the prepared titration solution into thereaction base solution, and when the molar ratio of lithium hexafluorophosphate to oxalic acid is 1:1.8-1:2.5 and the reaction temperature is 15-40 DEG C, carrying out a reaction to generate the lithium tetrafluorooxalatophosphate; when the molar ratio of lithium hexafluorophosphate to oxalic acid is 1:1.8-1:2.5 and the reaction temperature is 15-40 DEG C, carrying out a reaction to generate the lithium difluorodioxalatophosphate; after the reaction is finished, adjusting the temperature of the reaction system, continuously stirring the reaction product for a period of time, and filtering andrecrystallizing the reaction product to obtain the required products. The method is mild in reaction condition and easy to control, the solvent can be repeatedly used, the cost is saved, and the yieldis further increased.
Owner:武汉海斯普林科技发展有限公司
Solid chlorine dioxide anti septic
A solid ClO2 disinfectant for the drinking water, food and medical apparatus is prepared through mixing oxalic acid with citric acid, grinding, adding dextrin, activating, mixing with sodium chlorite, aluminium chlorate, glucose and sodium chloride, and packing.
Owner:兰州金陵石化有限责任公司
Methods and compositions for wet etching
InactiveUS20080041813A1Efficient removalAvoiding undercut and over-etchingSemiconductor/solid-state device manufacturingCable/conductor manufactureEtchingAmmonium persulfate
A composition comprising an aqueous solution of: a wet-etch formulation that is proven to etch copper; and a wetting agent. Exemplary wet-etch formulations include a mixture of a strong inorganic acid, such as sulfuric acid or hydrofluoric acid, and an oxidizing agent such as hydrogen peroxide, and further include ammonium persulfate. Exemplary wetting agents include organic acids such as citric acid, acetic acid, oxalic acid, or formic acid. Processes of using the compositions for wet-etching, or chemical mechanical polishing, or fabricating thick copper inductors, are further provided.
Owner:ATMEL CORP
Tinned steel plate surface treatment solution and preparation method thereof
ActiveCN104357825AGuaranteed corrosion resistanceGood adhesionMetallic material coating processesChromium freePhosphate
The invention discloses a tinned steel plate surface treatment solution and a preparation method thereof. The surface treatment solution contains 2-25 g / L disodium hydrogen phosphate, 2-13 g / L sodium pyrophosphate, 1-15 g / L ammonium hydrofluotitanate, 1-10 g / L potassium fluosilicate, 2-14 g / L nickel nitrate, 1-12 g / L zirconium titanate, 1-10 g / L sodium metaaluminate, 5-12 g / L manganese dihydrogen phosphate, 2-5 g / L sodium phytate and 1-7 g / L oxalic acid. The preparation method comprises the following steps: a. adding the disodium hydrogen phosphate and sodium pyrophosphate into deionized water, and stirring; b. adding the ammonium hydrofluotitanate, potassium fluosilicate, nickel nitrate, zirconium titanate, sodium metaaluminate and manganese dihydrogen phosphate; and c. regulating the pH value of the solution to 3-5 with phosphoric acid. On the basis of chromium-free passivation, the surface treatment solution ensures the corrosion resistance of the tinned plate without degrading the sulfur resistance, and also has excellent paint adhesiveness.
Owner:深圳市九久电子科技有限公司
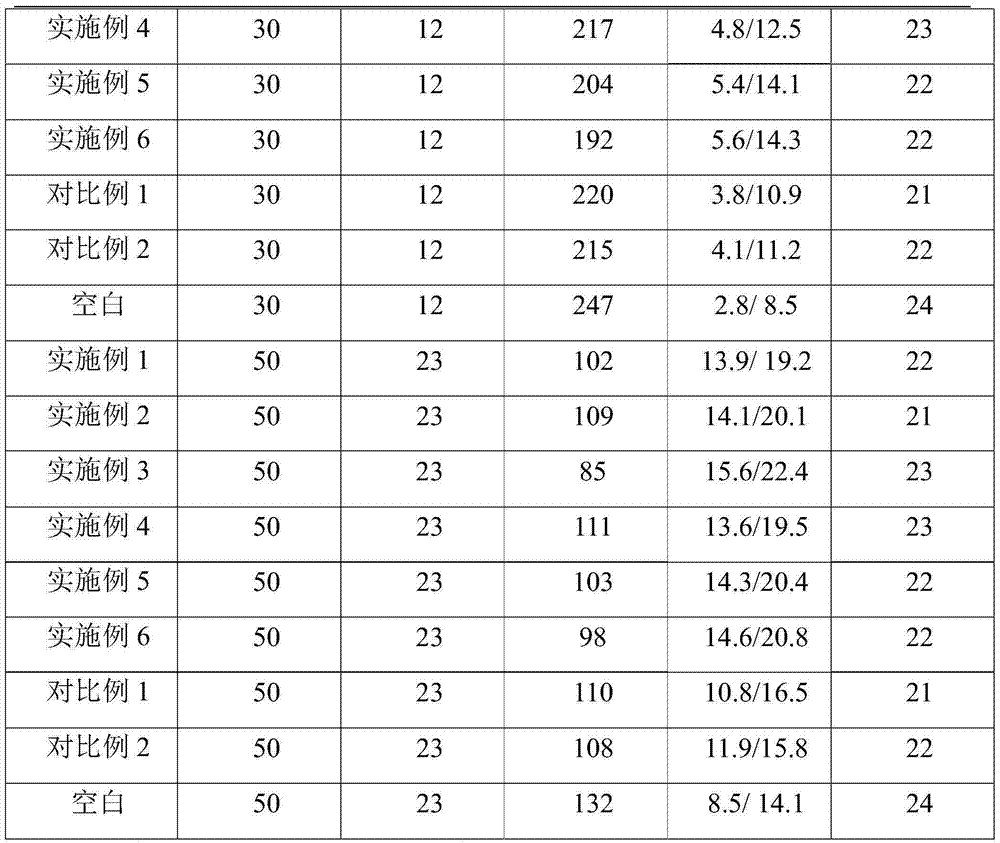
Oil well cement low-temperature composite early-strong coagulation accelerator
InactiveCN104774600AImprove early strengthImprove the coagulation effectDrilling compositionSodium acetateCalcium formate
The invention discloses an oil well cement low-temperature composite early-strong coagulation accelerator. The coagulation accelerator is prepared by mixing the following inorganic / organic components in parts by weight: 0.5 to 1 part of inorganic salt, 0.5 to 2 parts of organic salt, 0.04 to 0.1 part of organic amine, and 0.1 to 0.5 part of organic acid; wherein the inorganic salt is a mixture of calcium sulfate and sodium silicate; the organic salt is one or two of calcium formate, calcium oxalate, sodium acetate, and iron lactate; the organic amine can be diethanolamine, triethanolamine, or methanamide; and the organic acid is formic acid or oxalic acid. The provided coagulation accelerator has the advantages of low production cost and good early-strong effect, and also has a certain coagulation accelerating effect. The coagulation accelerator is suitable for international A-grade / G-grade oil well cement, and can be co-used with weighting materials (iron ore powder, barite powder, etc.) or lightening materials (fly ash, floating beads, etc.) to prepare cement mortar with different densities according to the needs.
Owner:CNPC BOHAI DRILLING ENG
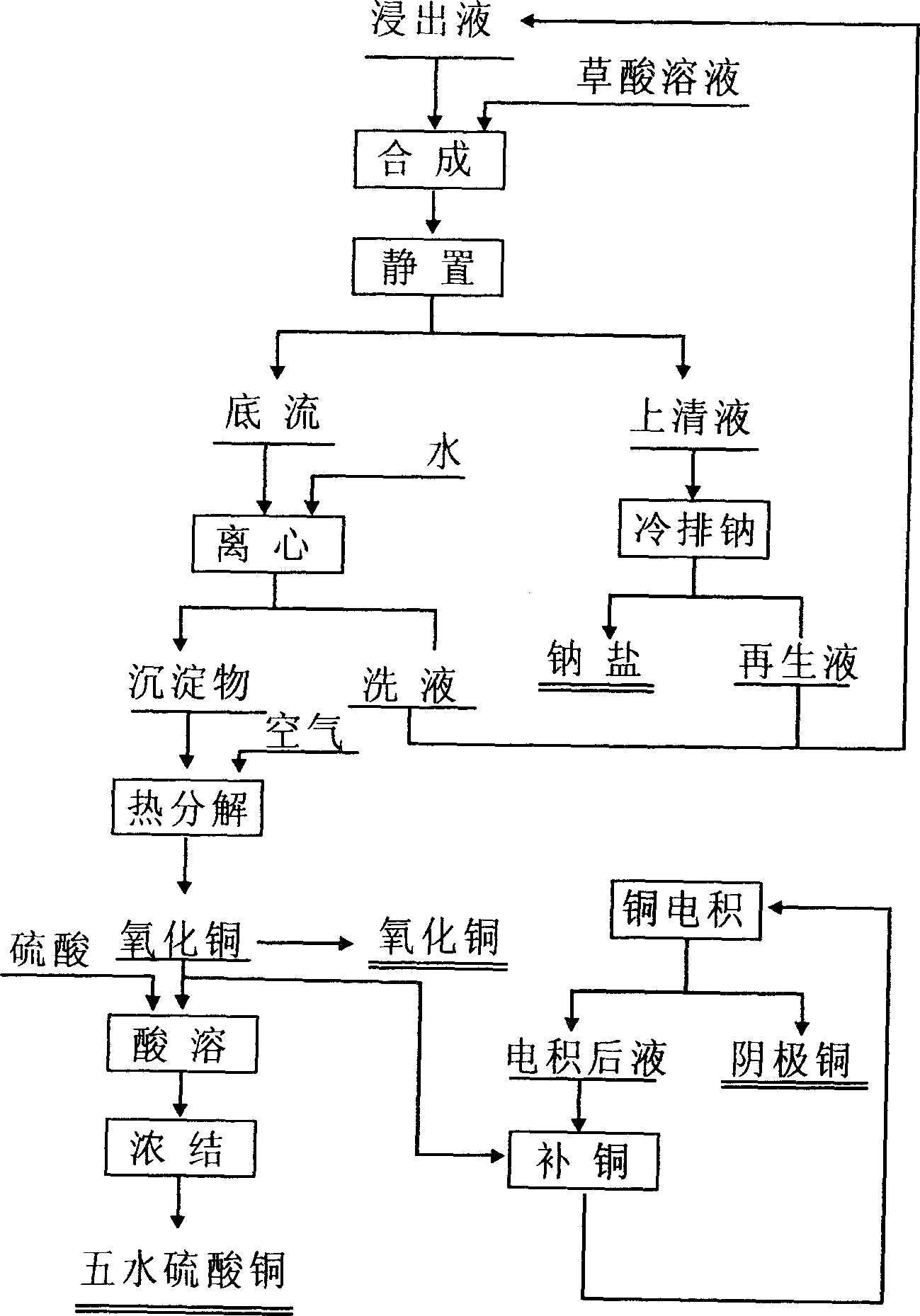
Method for extracting copper from organic silicon chemical waste residue leaching liquid of chloride and mixed salts containing copper
InactiveCN1844422AGuaranteed separation effectLow costPhotography auxillary processesCopper oxides/halidesChemical industryDecomposition
The invention selects oxalic acid solution to act as the precipitating agents of organosilicon chemical industry waste slag leachate of copper chlorate or mixture salt system, after filtering, washing and drying of the process residue of copper oxalic acid, heat decomposition in oxidizing atmosphere and at the temperature of 500~900 centigrade, the production of copper oxide is obtained by product yield cool-down. The yield acid of oxalic acid precipitation process is retuned organosilicon chemical industry waste slag leachate system to act as infusion. After the heat decomposition copper oxide dissolves in sulphuric acid, the solution can be used in electrodepositing cathode copper or regenerating copper sulphate, also can be used to produce cathode copper by copper electrodepositing solution dissolution regeneration electrodeposition, also sell as commercial copper oxide.
Owner:范有志
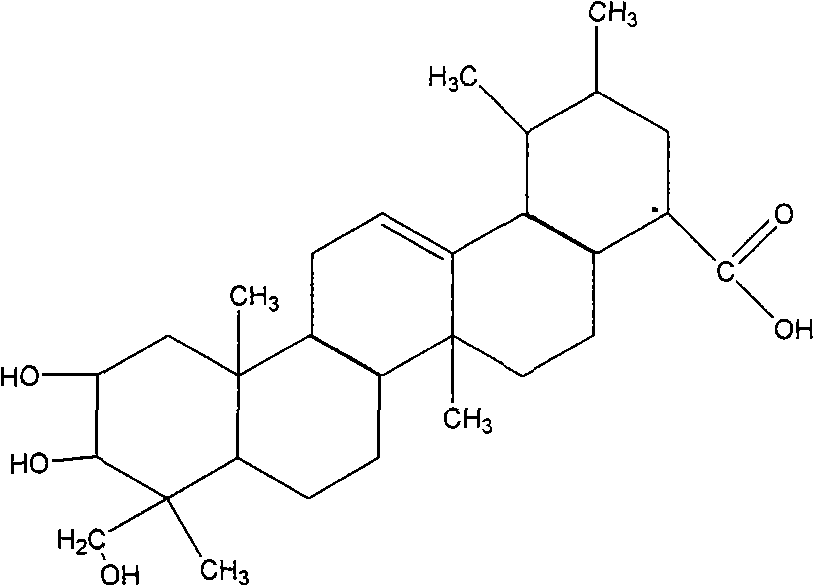
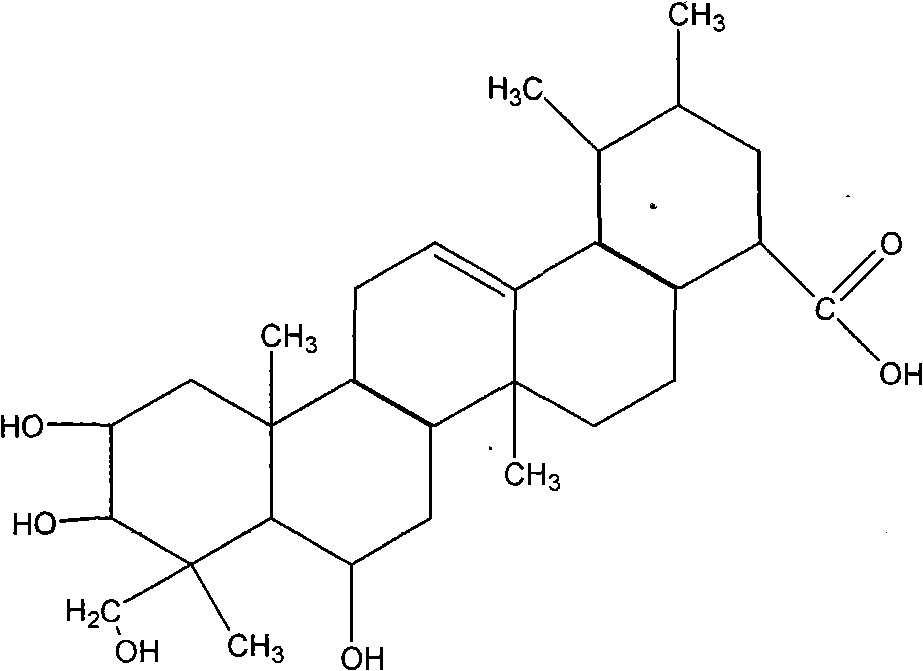
Application of asiatic acid and madecassic acid in preparation of alpha-glucosidase inhibitor drugs
Asiatic acid and madecassic acid are extracted and prepared from Centella asiatica (L.) Urban, and pharmacological experiments prove that the asiatic acid and the madecassic acid have the activity of inhibiting alpha-glucosidase. The invention discloses a new use of the asiatic acid and the madecassic acid in the preparation of alpha-glucosidase inhibitor drugs, and the asiatic acid, the madecassic acid or a composition containing the asiatic acid and the madecassic acid can be used for preparing the alpha-glucosidase inhibitor drugs. The invention simultaneously discloses a new preparation method of the asiatic acid and the madecassic acid.
Owner:赵全成
Method for reducing arsenic in amine extracted germanium organic phase
ActiveCN101619390AMeet the requirements of deep processingProcess efficiency improvementFiltrationKerosene
The invention relates to a method for reducing arsenic in amine extracted germanium organic phase, which belongs to the technical field of non-ferrous metallurgy. Material containing germanium and arsenic is leached by sulfuric acid, arsenic reducing agent capable of reducing As5<+> into As3<+> is added into leaching solution according to 1-5g / l, and final acid PH is adjusted to 0.5-1.5; after desilication and filtration, germanium organic complexing agent is added into the solution according to the weight ratio of 8-12 of germanium organic complexing agent to germanium to increase the selective extraction of germanium; and germanium is extracted by a N235 kerosene system, obtained germanium organic phase is washed by oxalic acid solution with the weight percentage of 1-2 percent, and then alkalistripping and hydrolysis are carried out to prepare germanium concentrate. The Na2SO3 is preferably adopted as arsenic reducing agent, and tartaric acid is preferably adopted as the organic complexing agent. The method has the advantages that even the solution has high arsenic concentration, the arsenic content of the produced germanium concentrate can be reduced to below 1 percent to meet the requirements of deep processing of germanium.
Owner:YUNNAN WUXIN IND
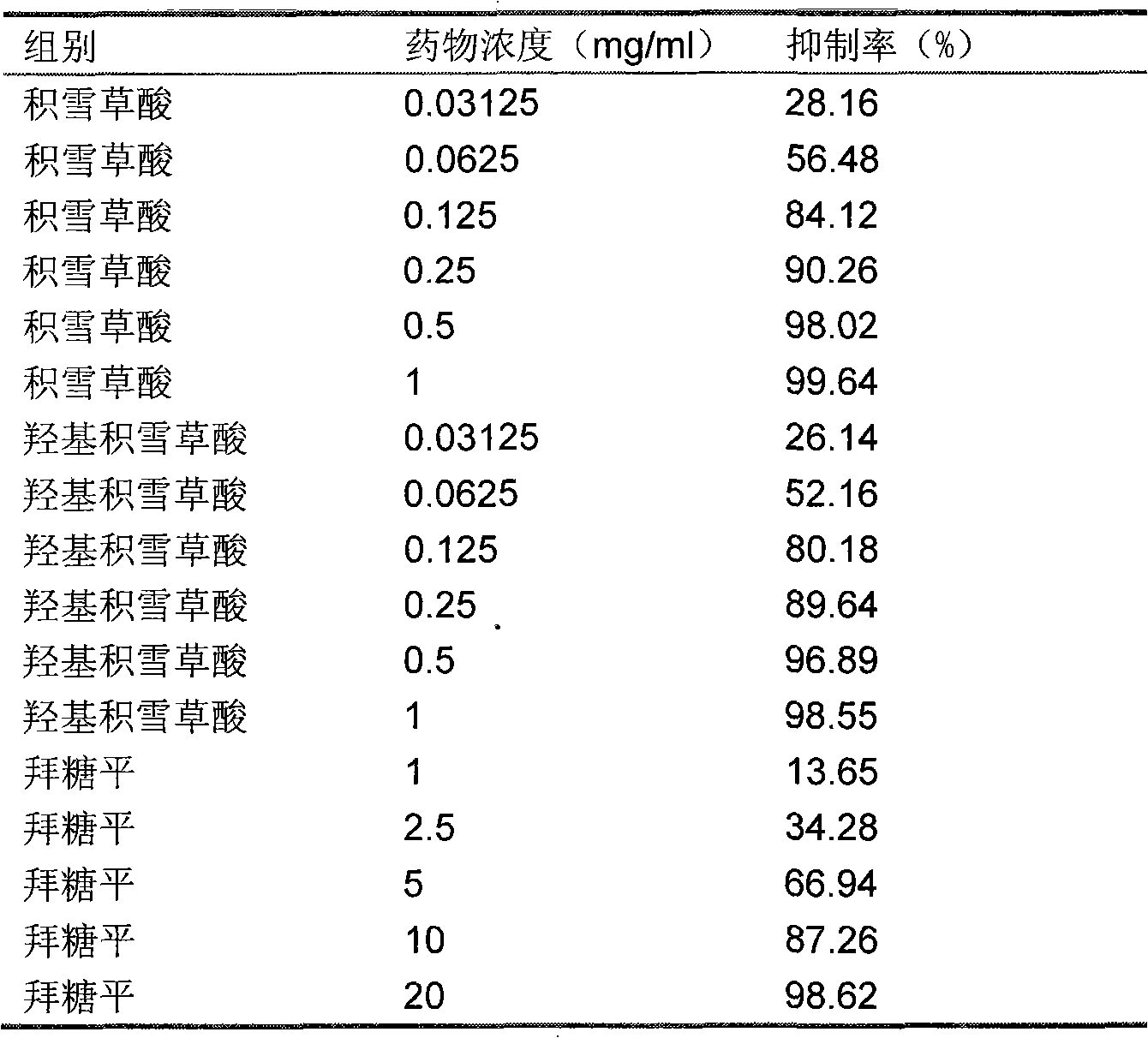
Modified 5A molecular sieve used for removing small-amount n-hexane from isohexane and regeneration method thereof
ActiveCN104888695AHigh porosityLarge adsorption capacityOther chemical processesCombustible gas purificationOxalateMolecular sieve
Disclosed is a modified 5A molecular sieve used for removing small-amount n-hexane from isohexane. The 5A molecular sieve to be modified is soaked and dealuminized through the ethanol solution of oxalic acid, and the modified 5A molecular sieve is obtained accordingly. Meanwhile, the invention discloses a regeneration method of the modified 5A molecular sieve after the n-hexane is absorbed. The modified 5A molecular sieve and method have the advantages that the modified 5A molecular sieve has larger porosity, the absorption amount of the n-hexane is increased, and more isohexane (containing small amount of n-hexane) can be treated; by means of the vapor replacement method, carbon deposition due to local excessively high temperature when the n-hexane is removed through high temperature and pressure directly is avoided.
Owner:岳阳金瀚高新技术股份有限公司
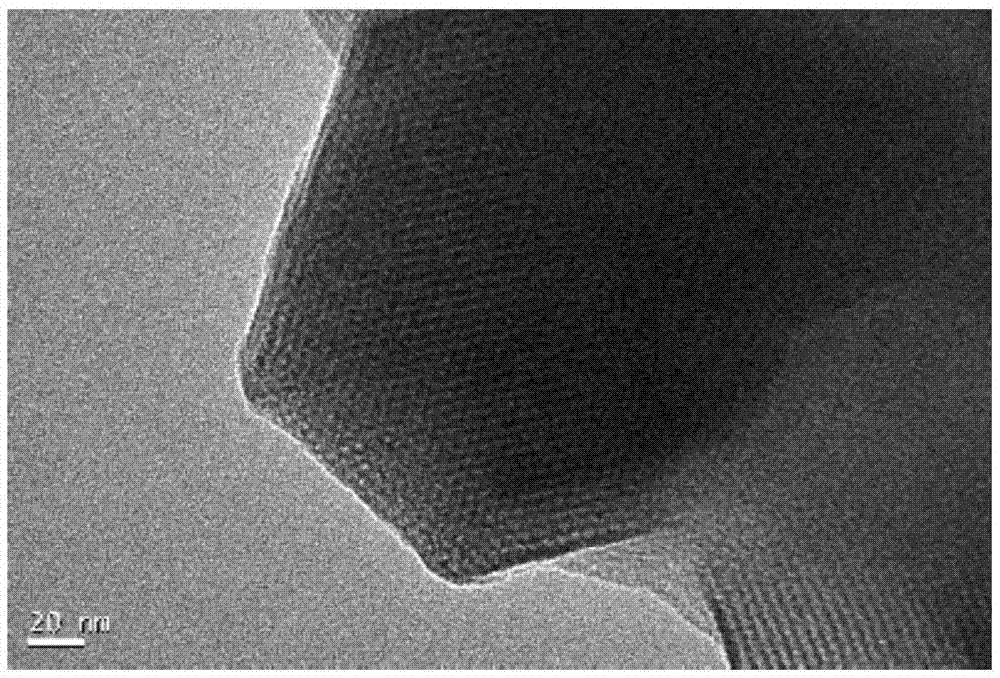
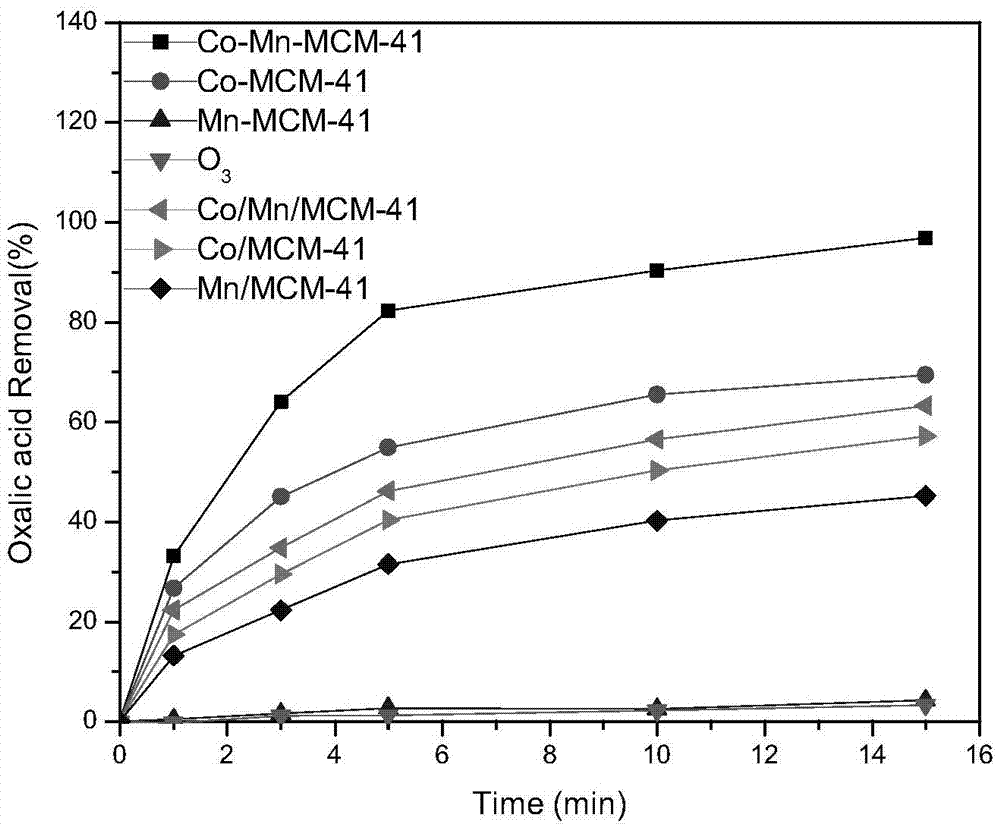
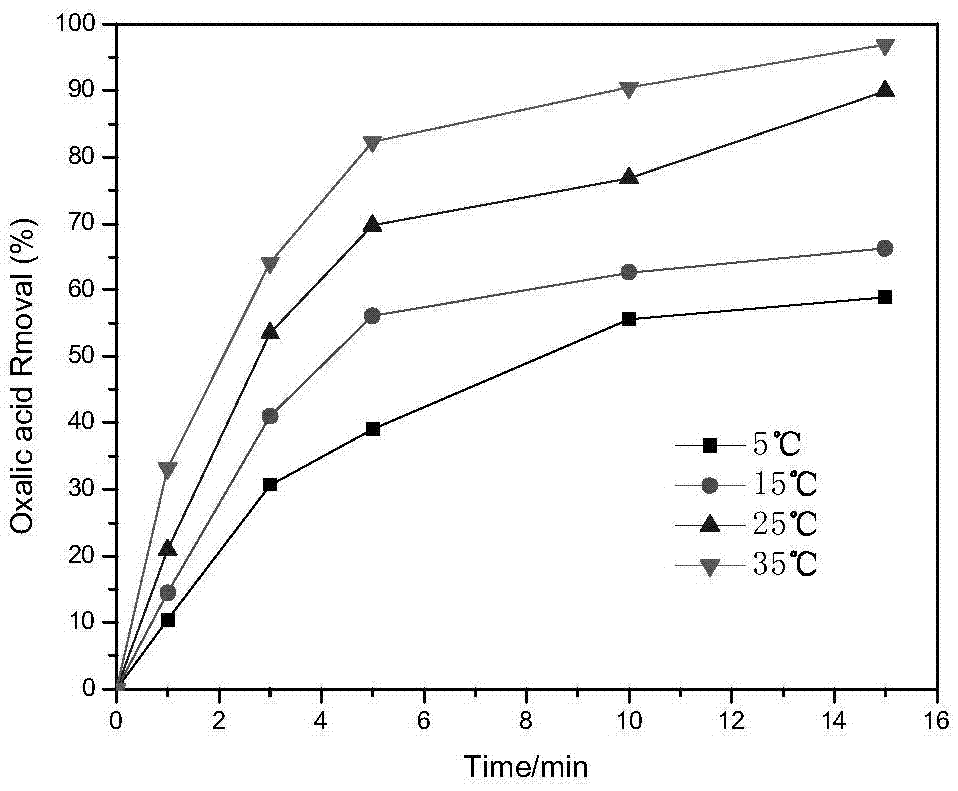
Preparation method and application of Co-Mn-MCM-41 molecular sieve
InactiveCN105435829AGood catalyticShort reaction timeMolecular sieve catalystsWater contaminantsOxalateCacodylic acid
The invention discloses a preparation method of a Co-Mn-MCM-41 molecular sieve. The preparation method comprises the following steps: dissolving sodium silicate used as a silicon source, cobalt salt used as a cobalt source, manganese salt used as a manganese source and a cationic surface active agent used as a template agent in water so as to form a mixture; and crystallizing under a strong alkaline condition, separating, washing and drying a crystallized product, then calcining the crystallized product so as to remove the template agent, thereby obtaining the Co-Mn-MCM-41 molecular sieve. The molecular sieve provided by the invention has a highly ordered one-dimensional hexagonal mesopore structure, and is large in specific surface area and high in catalysis activity; and results of an experiment of removing oxalic acid by ozonation catalysis indicate that through 15-minute catalysis based on the molecular sieve, the oxalic acid removal rate can be up to 97% and is 24 times that of ozonation under a catalyst-free condition.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Catalyst used in catalytic oxidation of glyoxal for preparing glyoxylic acid and preparation method of catalyst
InactiveCN102553628ALow priceHigh selectivityMolecular sieve catalystsOrganic compound preparationOxalateGlyoxylic acid
The invention provides a catalyst used in catalytic oxidation of glyoxal for preparing glyoxylic acid. The catalyst is V2O5 / SBA-15, wherein SBA-15 is used as a carrier to load V2O5. According to the preparation method, the SBA-15 is used as the carrier, oxalic acid solution of ammonium metavanadate is used as impregnation precursor, and the catalyst is prepared by using an equivalent-volume impregnation method. Compared with the noble metal catalyst, the V2O5 / SBA-15 provided by the invention is low in price, the glyoxylic acid can be obtained from the glyoxal under the action of the V2O5 / SBA-15 catalyst without maintaining a certain pH value of a reaction system; only a small amount of oxalic acid is detected from a product; and the catalyst has high glyoxylic acid selectivity and less byproducts.
Owner:TAIYUAN INST OF TECH
Production method for solar cell high-density monodisperse silver powder
The invention discloses a production method for solar cell high-density monodisperse silver powder. According to the production method, glyoxal serves as a reducing agent for reducing silver nitrate, and glycolic acid and oxalic acid generated by glyoxal reaction serve as components of silver powder dispersing protective agents. While flow concurrence is performed in a reactor, a glyoxal solution, a sodium hydroxide solution and a silver nitrate solution are added into the reactor, and the molar ratio of the silver nitrate, the glyoxal and sodium hydroxide is controlled to be 1:0.25-0.35:1-1.4, the pH value of a reaction solution is kept at 8-10, the reaction temperature is kept at 20 DEG C-40 DEG C, after feed intake is finished, stirring and reacting are continued for 0.5 hour-1 hour, and therefore reaction is complete, and silver crystal grain crystallization is complete. Silver powder particles are approximately spherical, the surfaces are smooth and glossy, the particle size distribution is narrow, the average particle size is 1.2 micrometers-1.8 micrometers, the apparent density is 2.0 g / cm<3>-2.5 g / cm<3>, the tap density is 4.5 g / cm<3>-5.0 g / cm<3>, and the molar yield is 97-99.5%. The consumption of the reducing agent is small in the process of producing the silver powder, production cost is low, safe and environmentally friendly effects are achieved, the reaction condition is easy to control, product quality is stable, and industrialized application is easy.
Owner:TIANJIN VOCATIONAL INST
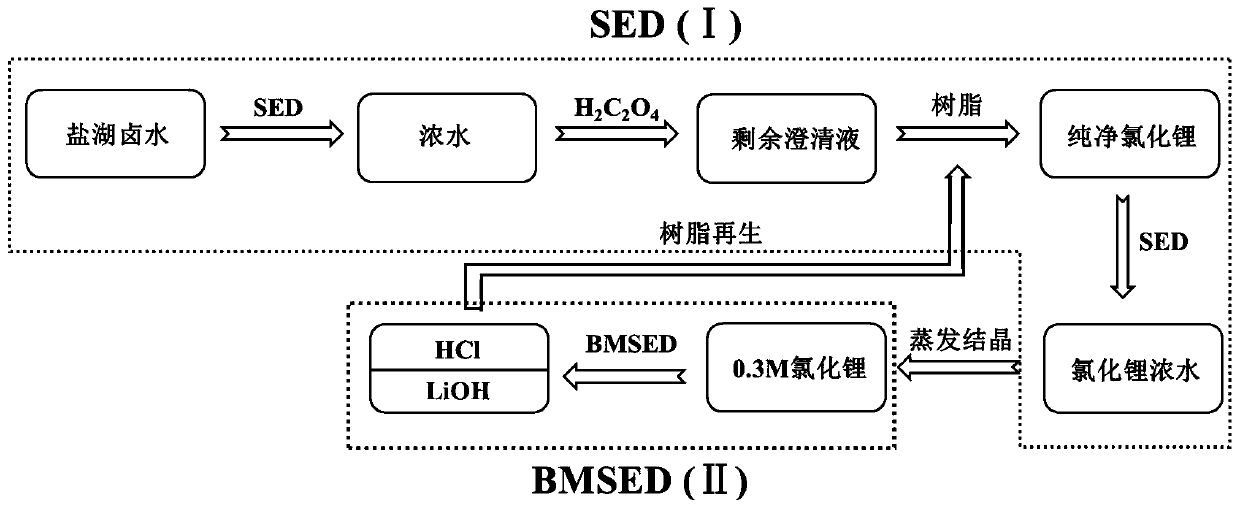
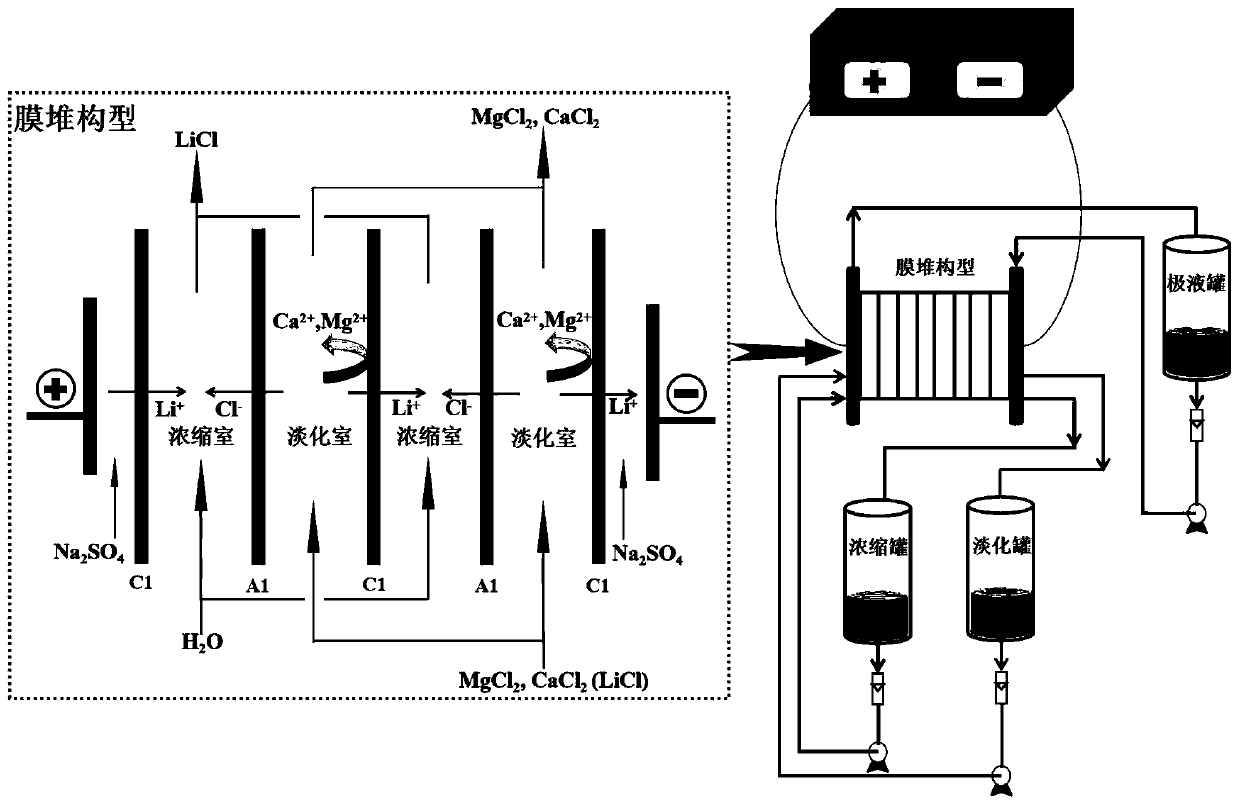
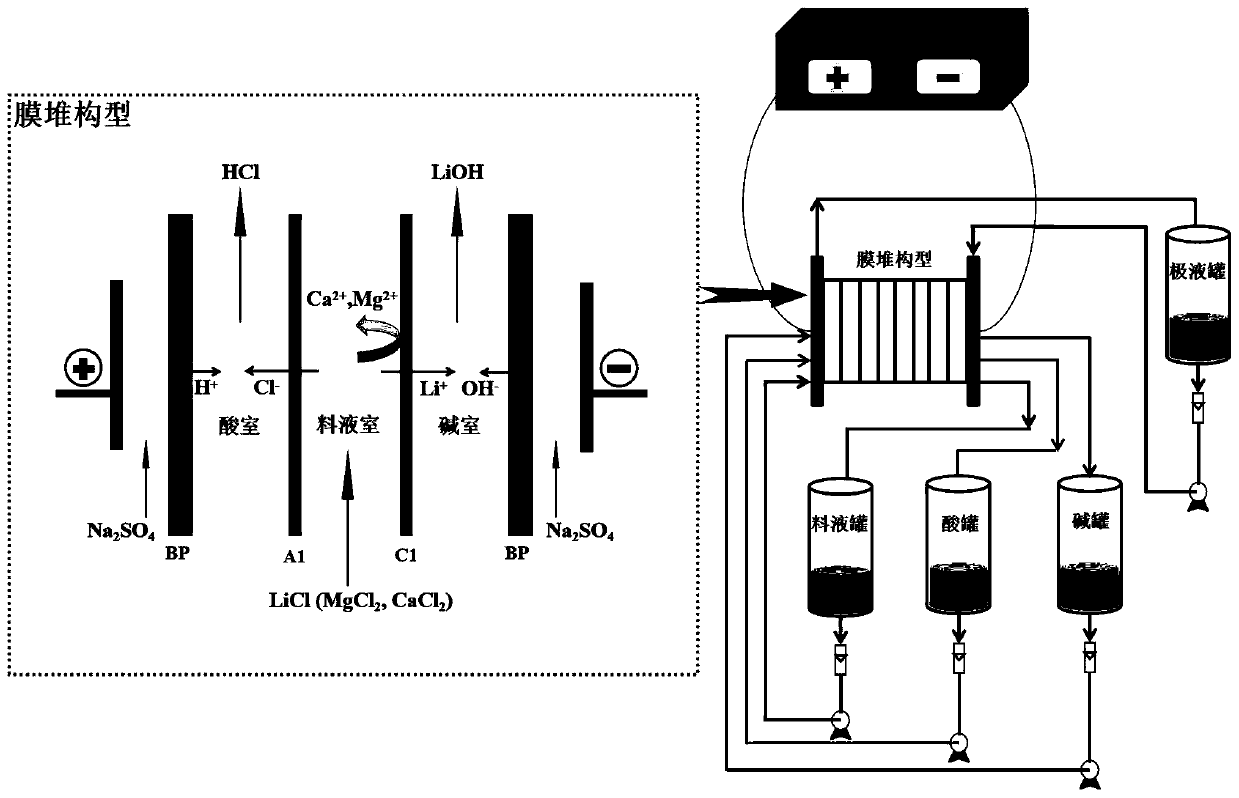
Method of integrating selective electrodialysis and selective bipolar membrane electrodialysis to treat salt lake brine to prepare lithium hydroxide
ActiveCN110065958AHigh purityReduce the impactLithium oxides/hydroxidesPreparation from chloridesLithium chlorideLithium hydroxide
The invention discloses a method of integrating selective electrodialysis and selective bipolar membrane electrodialysis to treat salt lake brine to prepare lithium hydroxide. The method comprises thefollowing steps of (1), introducing the salt lake brine with high calcium magnesium content into a univalent selective electrodialysis device to treat the salt lake brine; (2), adding oxalic acid into the brine obtained in step (1) to treat the brine; (3), treating a mother liquor obtained in step (2) with weak acid cation chelating resin; (4), introducing the brine obtained in step (3) into theunivalent selective electrodialysis device to concentrate lithium ions and meanwhile to further reduce the content of magnesium ions; (5), vaporizing the lithium-containing brine obtained in step (4)to obtain lithium chloride solids; (6), preparing the lithium chloride solids obtained in step (5) into a lithium chloride aqueous solution, and adding the lithium chloride aqueous solution into a univalent selective bipolar membrane electrodialysis device to prepare lithium hydroxide and a hydrochloric acid solution. The method and technology are easy to operate, the production cost, pollution tothe environment and energy consumption are greatly reduced, and the purity of produced lithium hydroxide is high.
Owner:ZHEJIANG UNIV OF TECH
Porous lithium iron phosphate/carbon composite microspheres and preparation method thereof
InactiveCN103078114ALarge specific surface areaHigh tap densityCell electrodesPhosphorus compoundsCarbon compositesIron salts
The invention provides porous lithium iron phosphate / carbon composite microspheres and a preparation method thereof. The porous lithium iron phosphate / carbon composite microspheres and the preparation method thereof are characterized in that iron phosphate oxalate is used as a precursor and the preparation method comprises the following steps: (1) dispersing solution of iron salt and phosphate solution into a precipitating agent ethanol, adding lithium salt, carrying out ultrasonic stirring to enable the solution of iron salt, the phosphate solution and the lithium salt to be totally dissolved, and adding a certain quantity of oxalic ethanol solution into the obtained solution; (2) placing the obtained mixture into an oven to dry at a certain temperature so as to obtain a yellow jelly iron phosphate oxalate precursor; and (3) after mixing a carbon source and the iron phosphate oxalate precursor, calcining to obtain the porous lithium iron phosphate / carbon composite microspheres. The preparation method provided by the invention is low in cost and is simple and easy to operate; the pH does not need to be regulated; the products have high purity, high tap density and good repeatability; the products are spherical particles formed by self-assembling disk nano-scale particles, and thus, the specific surface area is large; and moreover, the diffusion path of lithium ions is greatly shortened, so that the porous lithium iron phosphate / carbon composite microspheres have excellent electrochemical property and are suitable for large-scale production.
Owner:QINGDAO UNIV OF SCI & TECH
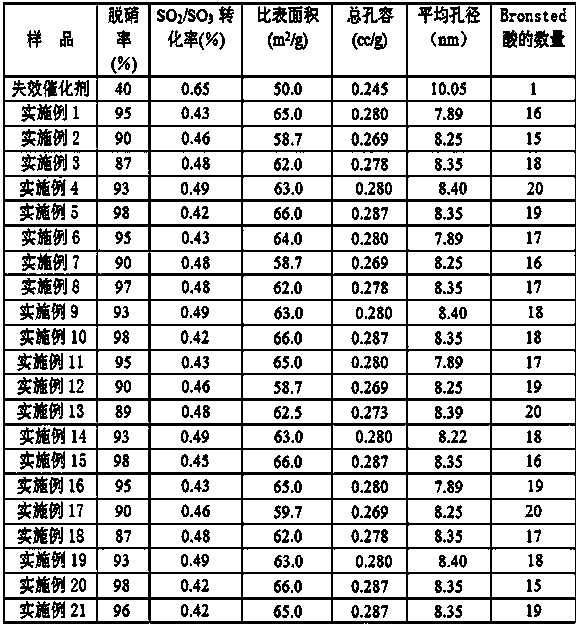
Cleaning liquid for regenerating denitration catalyst and cleaning method
ActiveCN104162456APromote sustainable developmentGuaranteed StrengthCatalyst regeneration/reactivationPhosphateSolvent
The invention discloses a cleaning liquid for regenerating a denitration catalyst. The cleaning liquid comprises a cleaning solution and a buffering solution, wherein the cleaning solution is formed by adding 1-10% of selective heavy metal catching agent HMCA-1 and 0.01-1% of polyoxyethylene ether non-ion surfactant into deionized water serving as a solvent; and the buffering solution is an orthophthalic hydrogen phthalate-dilute sulphuric acid solution, a hexamine-sulfuric acid solution, a sulfuric acid-ammonium sulfate solution, a phosphoric acid-ammonium monohydric phosphate or an oxalic acid-ammonium oxalate solution. The invention also discloses a method for cleaning a failed catalyst by using the cleaning liquid. The heavy metal catching agent HMCA-1 adopted by the cleaning liquid has an effect of selectively catching arsenic, mercury, calcium, beryllium, barium, magnesium, iron and the like on the surface of the catalyst; in addition, by virtue of the buffering solution, the quantity of Bronsted acid-acid sites of an SCR catalyst is greatly increased, so that a relatively small amount of ammonium metavanadate only needs to be added into a subsequent regenerated liquid; and the cost is lowered.
Owner:LUOYANG HAOHAI IND & TRADE
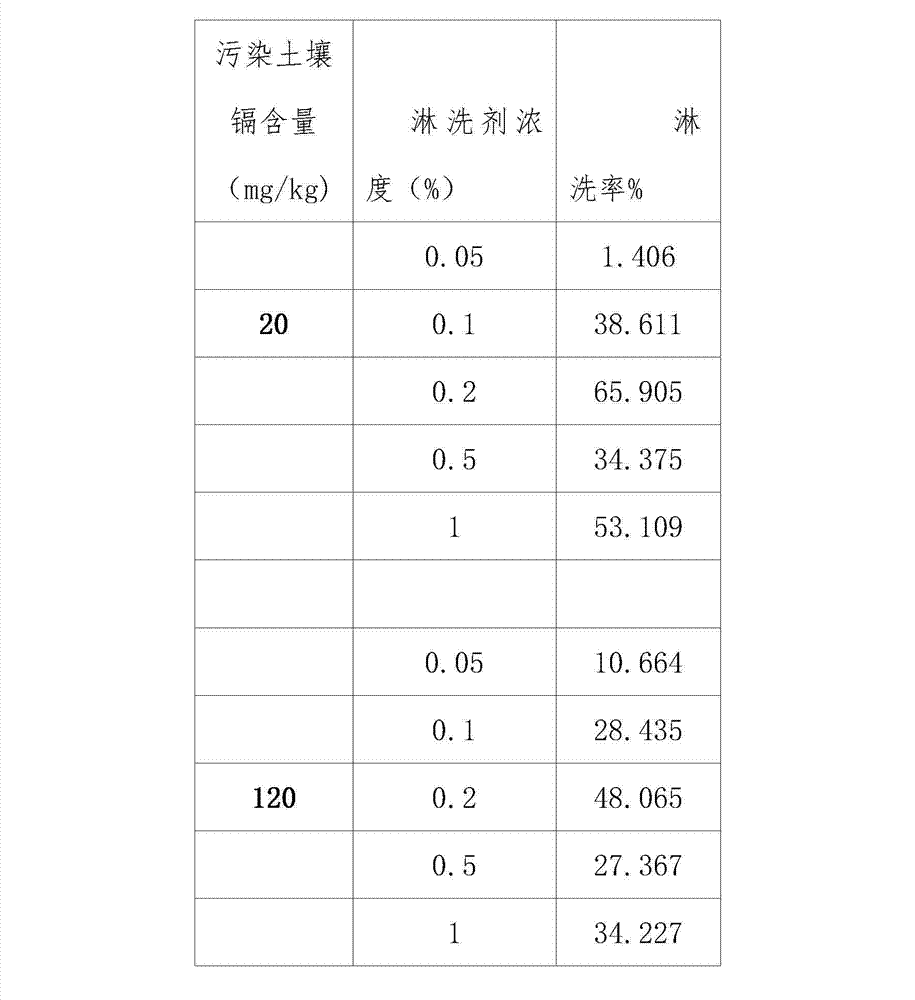
Application of oxalic acid in removal of soil cadmium pollution
The invention relates to an application of oxalic acid in removal of soil cadmium pollution. High-level pure oxalic acid is selected to prepare an oxalate solution with the concentration of 0.05wt%-1wt%, and the oxalate solution is used as an eluting agent; the eluting agent is added into polluted soil; an eluting process can be finished by oscillating, centrifuging and collecting an eluted solution at room temperature and then filtering the eluted solution; and a liquid to solid ratio of the chemical eluting agent to the polluted soil is 10:1 (ml / g). The chemical eluting agent formula is simple; the effect is outstanding; the operability is high; the application cost is low; the eluting rate is high; and a relatively good effect of removing cadmium ions in the soil is achieved. A test shows that when the concentration is 0.2 percent, the eluting rates are respectively 65.91 percent and 48.065 percent; and secondary pollution to the soil is avoided.
Owner:SICHUAN AGRI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com