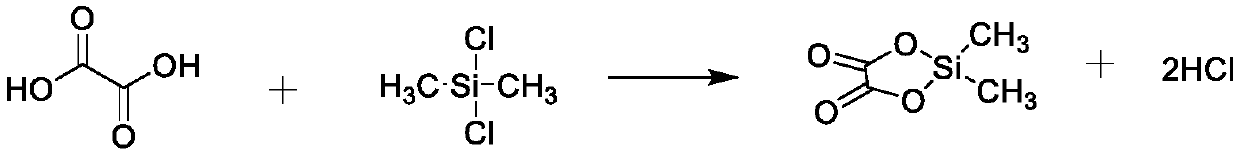Preparation method of lithium difluorobisoxalate phosphate solution
A technology of lithium difluorobisoxalate phosphate and lithium hexafluorophosphate is applied in the field of lithium ion batteries, which can solve the problems of unavailability, unaccurate control of reactions, industrialization impact, and the like, and achieves the effect of a simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a nitrogen glove box with a moisture content of less than 20 mass ppm, prepare a 1000ml three-neck flask containing a stirring bar, and add 500 g of diethyl carbonate with a moisture content of 10 mass ppm or less and 20.0 g of oxalic acid with a moisture content of 200 mass ppm into a three-necked flask, and seal the three-necked flask. Transfer the aforementioned three-necked flask to the outside of the glove box, put it in an oil bath at a temperature of 65°C, install a condenser tube, a constant pressure dropping funnel, insert a bubbler and use nitrogen gas to bubble slowly, and the bulging gas is absorbed by water . Using a magnetic stirrer, stir the solution well until a homogeneous solution is formed.
[0035] Next, 43 g of dichlorodimethylsilane was added into the constant pressure dropping funnel, and the dichlorodimethylsilane was dropped into the homogeneous solution in the three-necked flask for 30 minutes. After the dropwise addition, the constant pre...
Embodiment 2
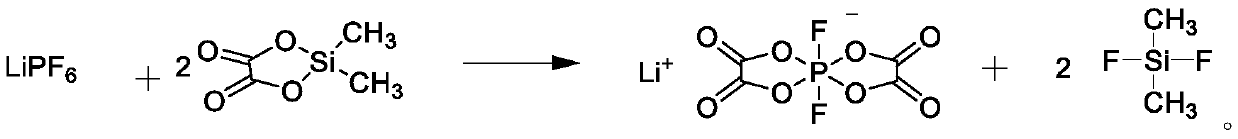
[0041] Using 51.6 g of dichlorodimethylsilane, the molar ratio to oxalic acid is 1.8, and the non-aqueous solvent uses dimethyl carbonate, except that it is synthesized in the same manner as in Example 1, and is carried out in the same manner as in Example 1 19 Determination of F NMR, determination of chloride ion concentration, determination of free acid concentration. The product concentration in the solution calculated by NMR was 3.9% by mass, and the yield of lithium difluorobisoxalate phosphate calculated by lithium hexafluorophosphate was 75%. The chloride ion concentration was 4.9 mass ppm, and the hydrofluoric acid was 142 mass ppm.
Embodiment 3
[0043] Using 37.3 g of dichlorodimethylsilane, the molar ratio to oxalic acid is 1.3, and the non-aqueous solvent uses dimethyl carbonate, except that it is synthesized in the same manner as in Example 1, and is carried out in the same manner as in Example 119 F NMR determination, chloride ion concentration determination, free acid concentration determination. The product concentration in the solution calculated by NMR was 3.9% by mass, and the yield of lithium difluorobisoxalate phosphate was 76% based on lithium hexafluorophosphate. The chloride ion concentration was 1.4 mass ppm, and the hydrofluoric acid was 89 mass ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com