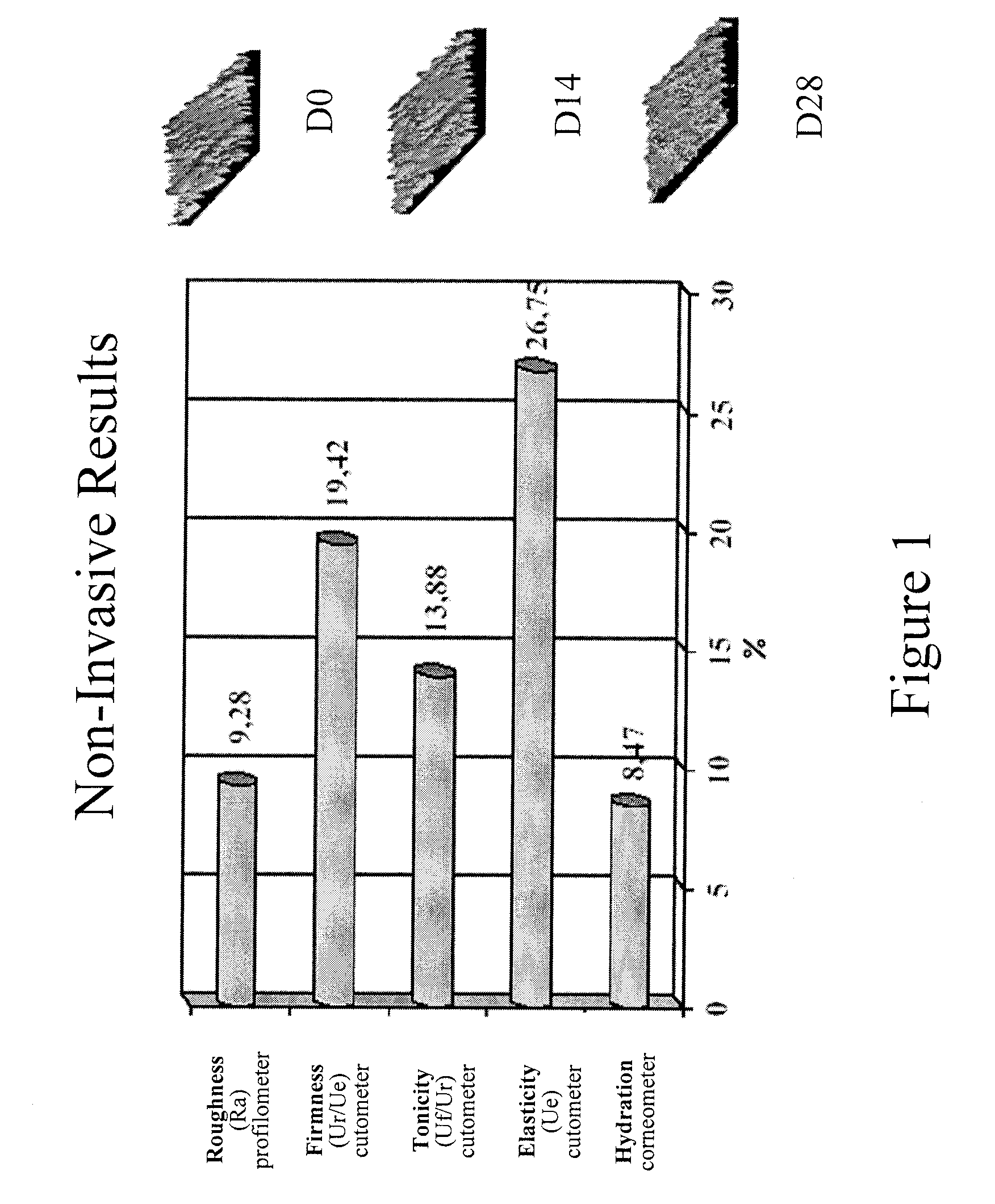
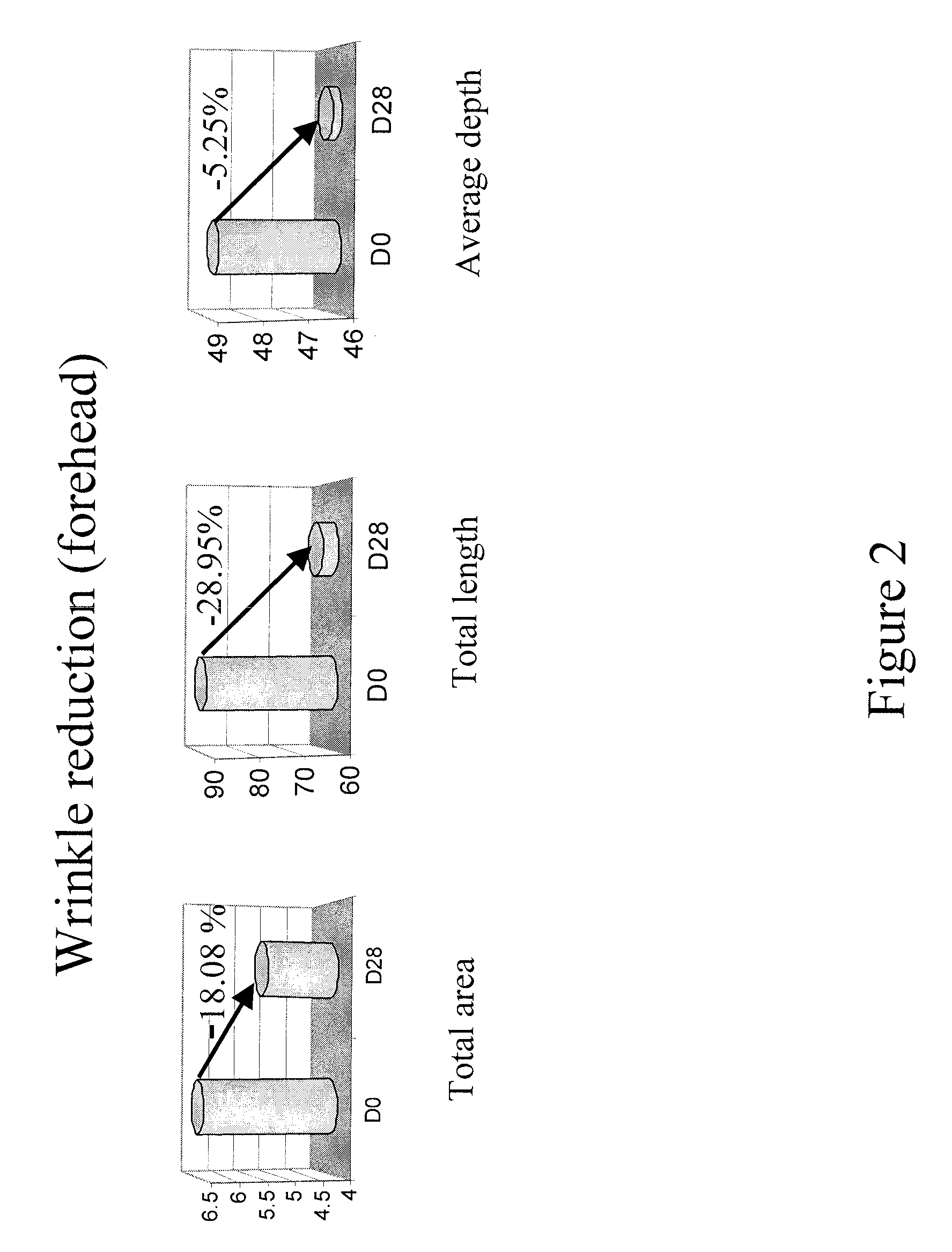
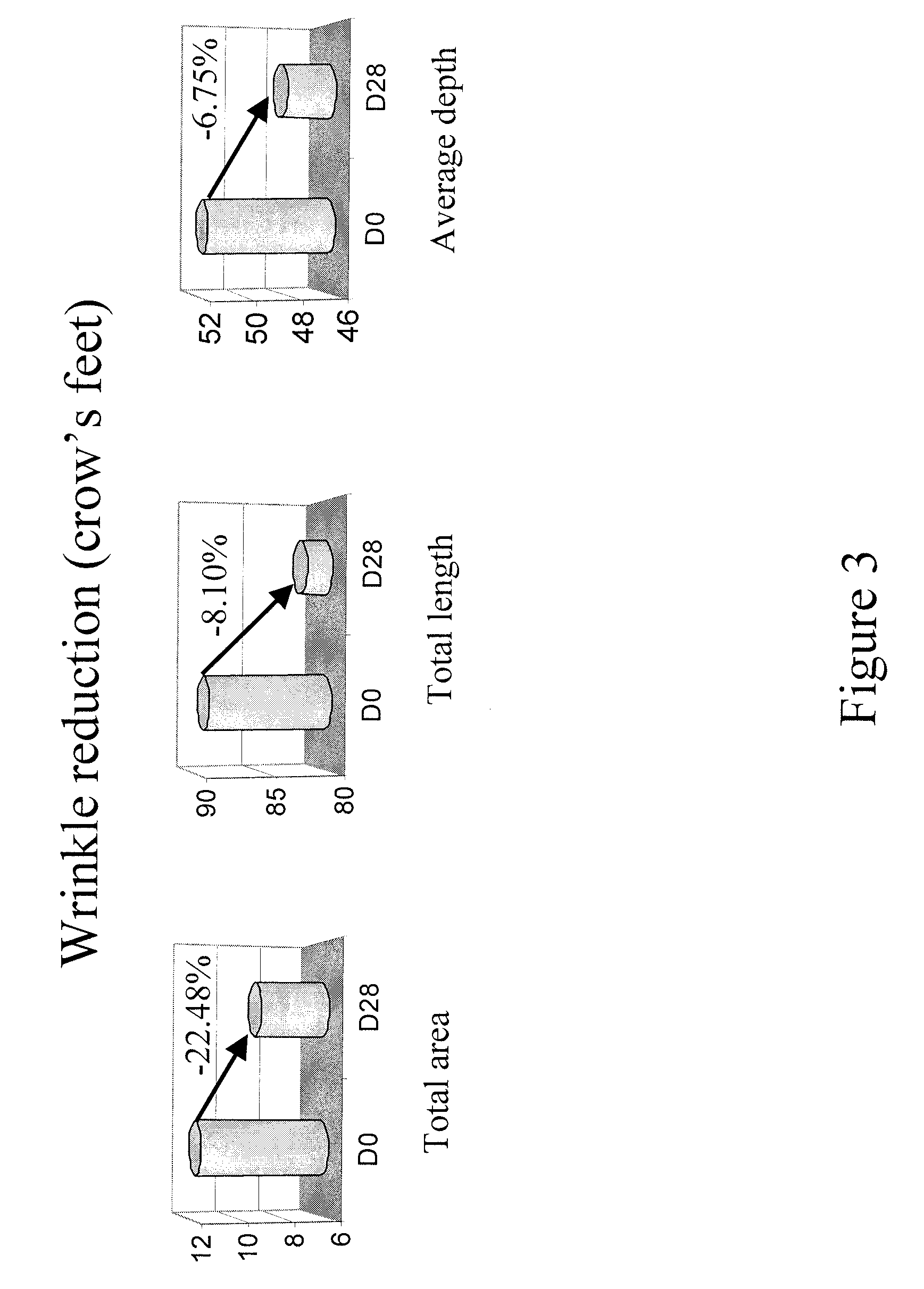
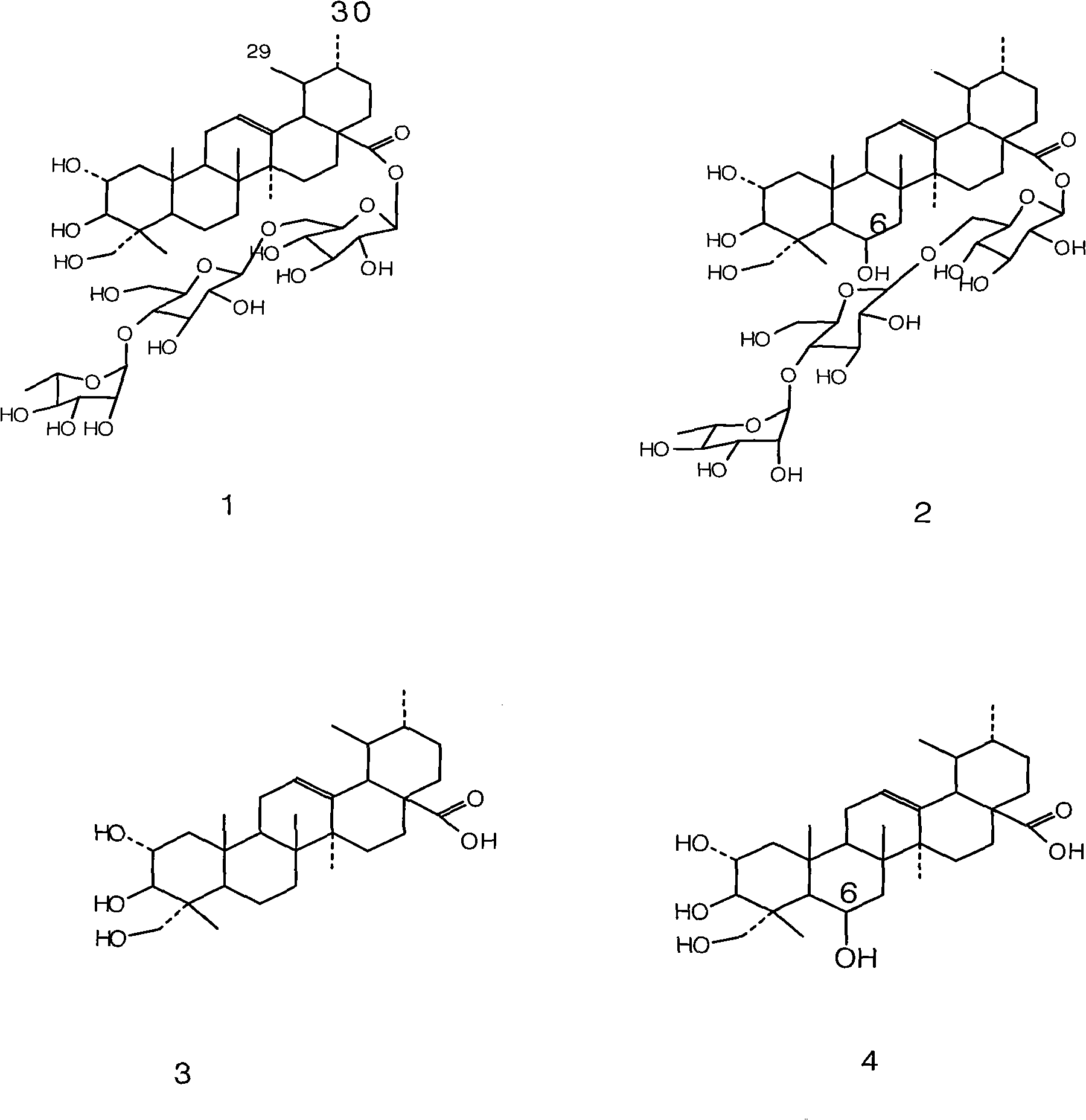
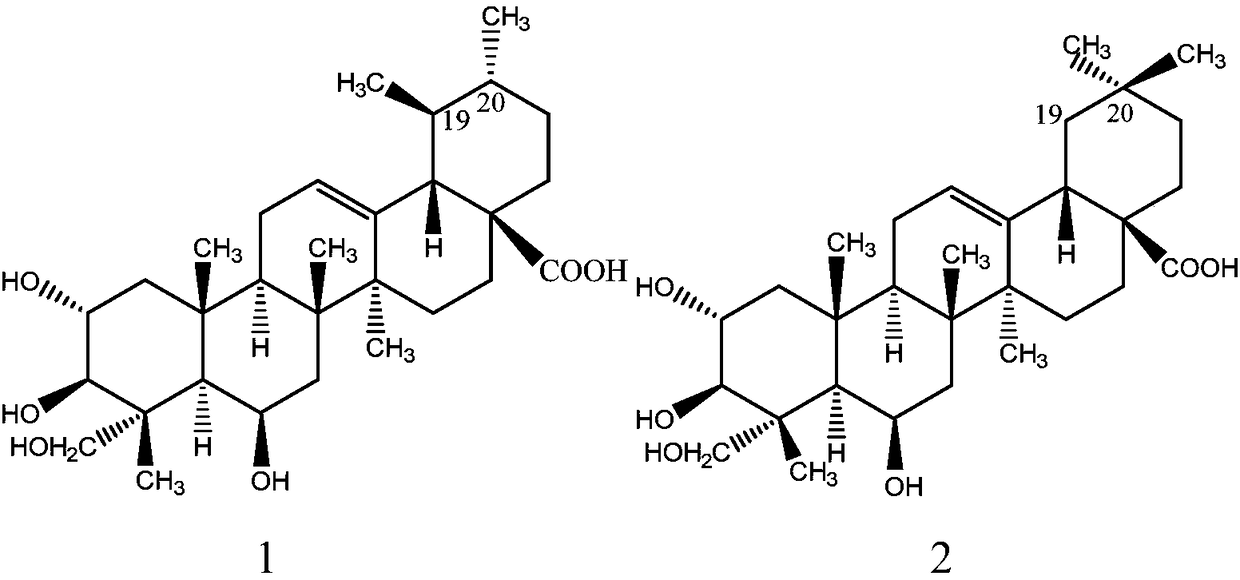
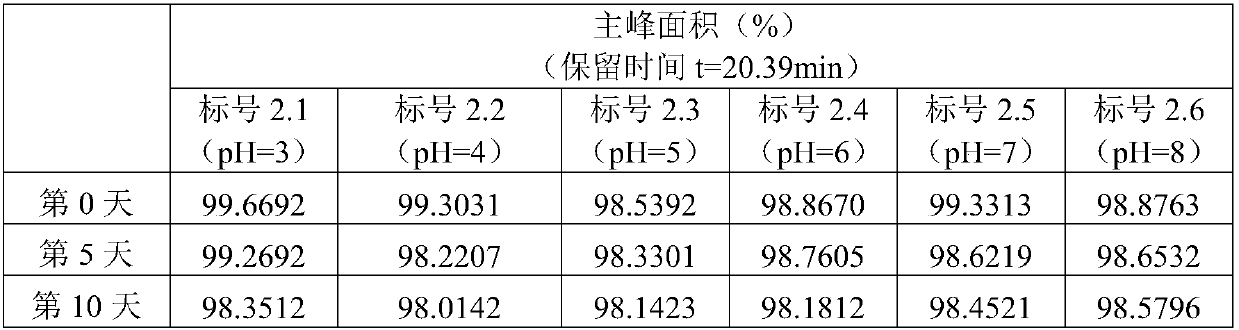
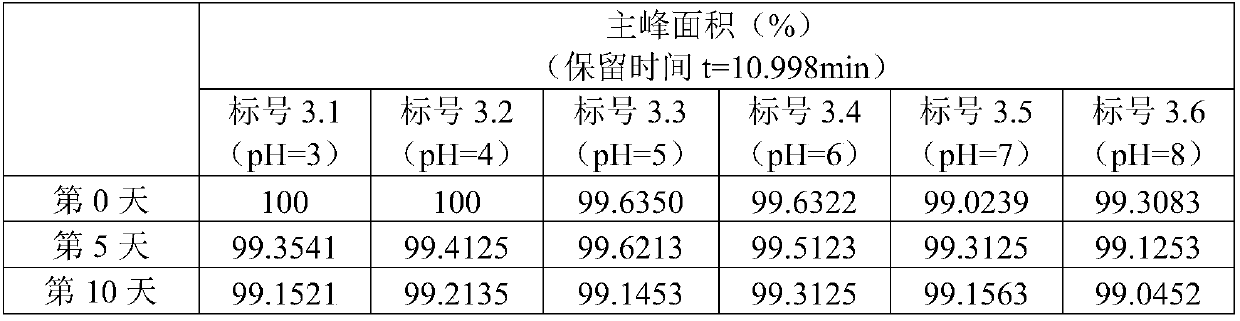
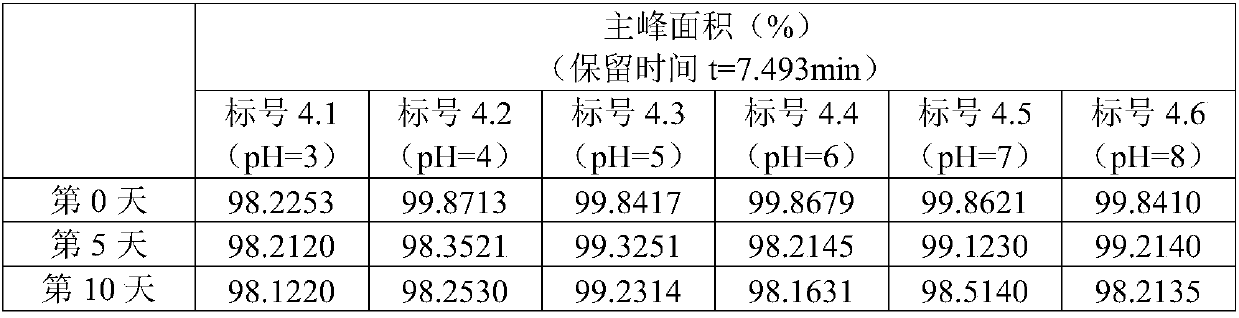
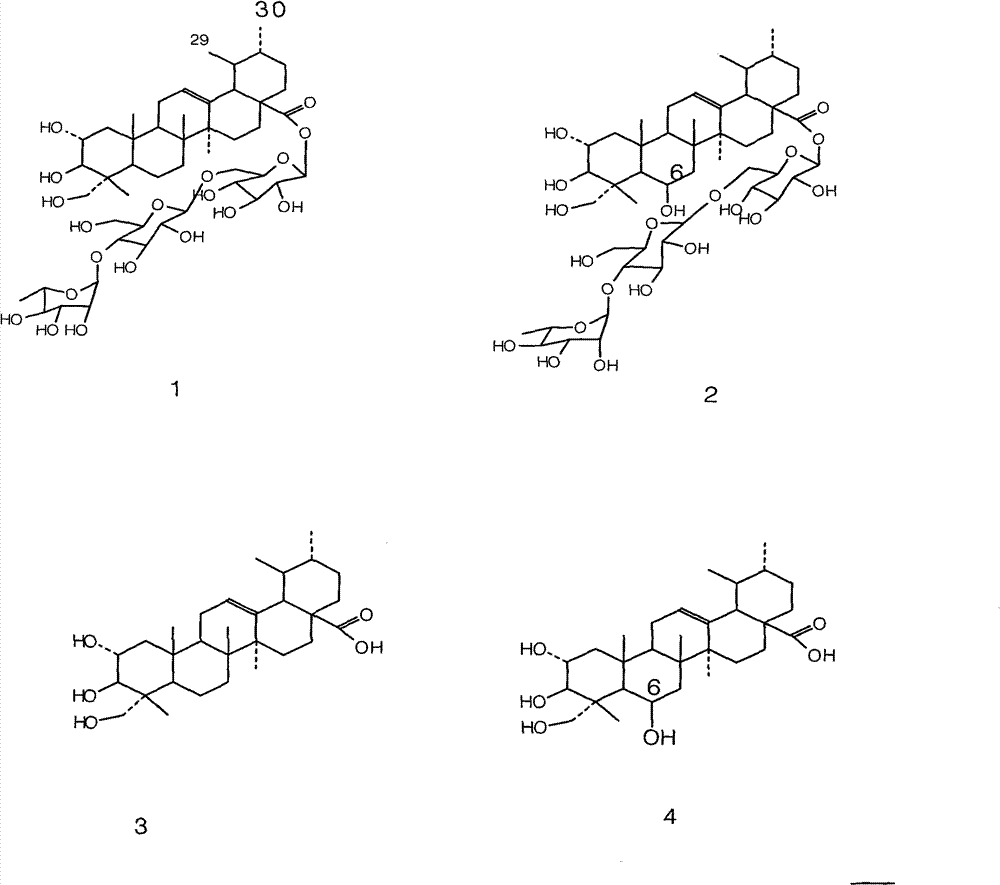
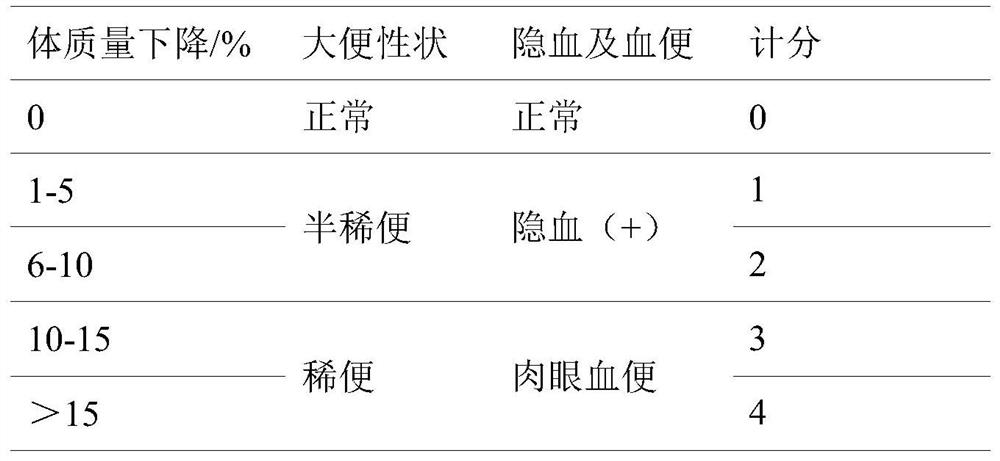
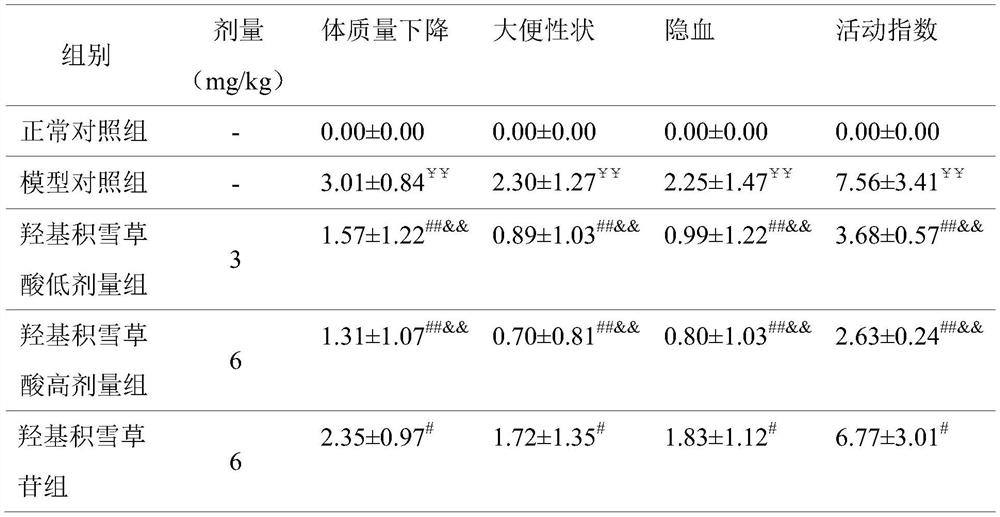
Patents
Literature
36 results about "MADECASSIC ACID" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anti-aging composition, kit and method of use
InactiveUS20070237735A1Reducing and preventing age-related skin symptomReducing and preventing symptomBiocideCosmetic preparationsMADECASSIC ACIDAntioxidant
An anti-aging composition comprising: (i) at least one biomimetic oligopeptide having a sequence of 20 amino acids or less; (ii) at least one lipoaminoacid; (iii) at least one pentacyclic triterpenoid selected from the group consisting of asiaticoside, madecassic acid, asiatic acid and madecassoside; (iv) at least one antioxidant; and (v) tetrahydropiperine; a kit comprising such composition and a method of use.
Owner:LABES DERMO COSMETIK
Method for preparing total asiatic acid, asiatic acid and madecassic acid from asiatic pennywort herb and use of prepared product
InactiveCN101991624ASimple processLow costOrganic active ingredientsMetabolism disorderAsiatic pennywortMADECASSIC ACID
The invention relates to a method for preparing total asiatic acid, asiatic acid and madecassic acid from asiatic pennywort herb and use of a prepared product. The method comprises the following steps of: preparing asiaticoside from coarse powder of the asiatic pennywort herb; treating the asiaticoside by using alkali liquor, acidifying, extracting by using n-butyl alcohol, and recrystallizing byusing alcohol to prepare the total asiatic acid; and performing chromatography on the total asiatic acid to obtain the asiatic acid and the madecassic acid. Meanwhile, the total asiatic acid, the asiatic acid and the madecassic acid can be used for reducing postmeal glucose and preventing and treating diabetes. The method has the characteristics that the process is simple, the cost is low, and the method is suitable for promotion and large-scale production.
Owner:SHANGHAI NORMAL UNIVERSITY
Centella asiatica triterpenic acid single-glucopyranoside composition, its preparation method, its quantitative analysis method and its application
The invention relates to a centella asiatica triterpenic acid single-glucopyranoside composition which is composed of ursane madecassic acid single-glucopyranoside, madecassic acid single-glucopyranoside and oleanane chebuloside II, wherein the mass ratio of ursane madecassic acid single-glucopyranoside to madecassic acid single-glucopyranoside to oleanane chebuloside II is 1:0.5-2:0.1-1, and the sum of the mass percentage content of three components is not less than 50%. The composition takes a centella asiatica extract as a substrate, the centella asiatica extract is fermented and hydrolyzed by microbes of beta-glucosidase or microbes capable of generating beta-glucosidase, and extracted by n-butanol or separated and purified by macroporous adsorption resin. The invention also provides a quantitative analysis method which is a HPLC quantitative analysis for three components by adding a proper amount of mobile phase of beta-cyclodextrin. Experimental research of pharmacodynamics proves that the composition has substantial activity for inhibiting tumor cells and fibroblast, the composition can be used for treating tumor and scar hyperplasia.
Owner:SHANGHAI NORMAL UNIVERSITY
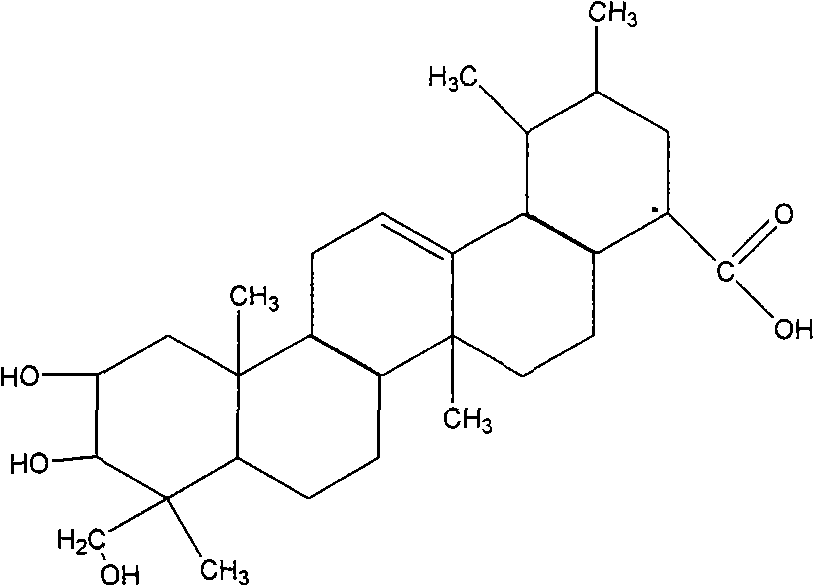
Application of asiatic acid and madecassic acid in preparation of alpha-glucosidase inhibitor drugs
Asiatic acid and madecassic acid are extracted and prepared from Centella asiatica (L.) Urban, and pharmacological experiments prove that the asiatic acid and the madecassic acid have the activity of inhibiting alpha-glucosidase. The invention discloses a new use of the asiatic acid and the madecassic acid in the preparation of alpha-glucosidase inhibitor drugs, and the asiatic acid, the madecassic acid or a composition containing the asiatic acid and the madecassic acid can be used for preparing the alpha-glucosidase inhibitor drugs. The invention simultaneously discloses a new preparation method of the asiatic acid and the madecassic acid.
Owner:赵全成
Salts of asiatic and madecassic acid suitable for the preparation of pharmaceutical and cosmetic compositions
InactiveUS6891063B1Facilitate their formulationIncrease topic bioavailabilityCosmetic preparationsSenses disorderMADECASSIC ACIDInjury mouth
Salts of asiatic and madecassic acid with pharmaceutically acceptable organic bases, suitable for the preparation of pharmaceutical and cosmetic compositions for the topical and systemic treatment of erithema, varicose ulcers, venous insufficiency, bedsores, delayed cicatrization, ambustions, traumatic and surgery wounds, alloeosises of the cutaneous trophism, ophthalmic alloeosises and inflammatory processes.
Owner:EUPHAR GRP SRL
Compositions Comprising Compounds Of Natural Origin For Damaged Skin
ActiveUS20100273729A1Improve antioxidant capacityEnhance anti-inflammatoryCosmetic preparationsBiocideQuercetinGlycyrrhizin
The present invention relates to a skin-protecting composition for the damaged skin, comprising glycyrrhizin, quercetin, rosmarinic acid, madecassic acid, chamazulene, bicalein and emodin. The composition of the present invention has all of excellent antioxidant, anti-inflammatory, wound-healing and moisturizing effects, thereby being widely used in medicine, cosmetic material or the like for the purpose of protecting the easily infectable, damaged and dried skin.
Owner:BIOSPECTRUM
Compositions comprising compounds of natural origin for damaged skin
ActiveUS7994141B2Improve antioxidant capacityEnhance anti-inflammatoryBiocideCosmetic preparationsWound healingMedicine
The present invention relates to a skin-protecting composition for the damaged skin, comprising glycyrrhizin, quercetin, rosmarinic acid, madecassic acid, chamazulene, bicalein and emodin. The composition of the present invention has all of excellent antioxidant, anti-inflammatory, wound-healing and moisturizing effects, thereby being widely used in medicine, cosmetic material or the like for the purpose of protecting the easily infectable, damaged and dried skin.
Owner:BIOSPECTRUM
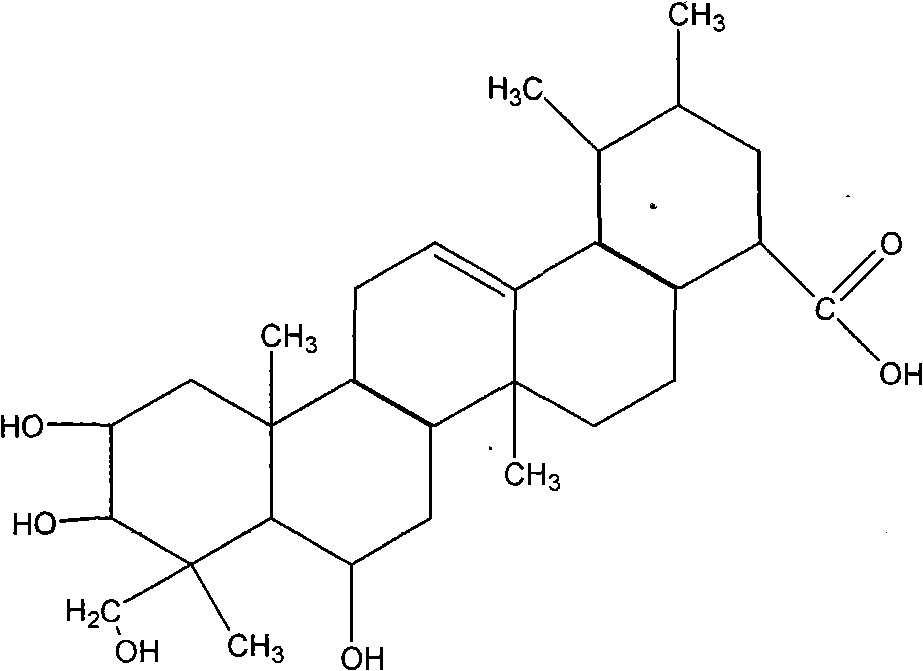
New application of madecassic acid
InactiveCN102641274AGood inhibitory effectShow curative effectOrganic active ingredientsSexual disorderDiseaseOral medication
The invention relates to a new application of madecassic acid, derivatives, and isomers thereof, and particularly relates to an application of madecassic acid and derivatives thereof, or compositions of madecassic acid or its derivatives and its isomers in the preparation of medicaments for prevention and / or treatment of breast-related diseases, or breast health-care products. Studies confirm that both oral administration and external use of madecassic acid or derivatives thereof or compositions of madecassic acid or its derivatives and its isomers have significant inhibition effect on breast hyperplasia of experimental rats.
Owner:苏州迪星生物医药科技有限公司
Method for extracting madecassic acid from herba centellae
The invention discloses a method for extracting madecassic acid from herba centellae. The method includes the following steps of firstly, smashing herba centellae, conducting reflux extraction through an ethyl alcohol solution with volume concentration of 60-70 v%, filtering an extraction solution, recovering solvent from filtrate, adding water at 40-50 DEG C for dissolving and filtering, and dissolving filter residues in an ethyl alcohol solution with volume concentration of 50-60 v% to obtain a crude extraction solution; secondly, adding rare-earth salts to the crude extraction solution to be mixed and evenly stirred to obtain mixed liquid; thirdly, applying the mixed liquid to an X-5 type macroporous resin column, conducting water washing till being colorless, conducting eluting through ethyl alcohol with volume concentration of 20-30 v%, conducting thin layer chromatography tracking and detecting, collecting ethyl alcohol eluent containing asiatic acid, conducting eluting through ethyl alcohol with volume concentration of 40-50 %, conducting thin layer chromatography tracking and detecting, collecting ethyl alcohol eluent containing madecassic acid, recovering ethyl alcohol, and conducting drying to obtain madecassic acid. The extracted madecassic acid is high in purity and high in yield.
Owner:桂林益天成生物科技有限公司
Topical skincare compositions comprising centella asiatica selected triterpenes
ActiveUS20190374591A1Safe and effective amountTreat symptomsCosmetic preparationsToilet preparationsTriterpeneMADECASSIC ACID
A topical skincare composition including a Centella asiatica extract including one or more triterpenes, wherein at least one of the one or more triterpenes is madecassic acid, an Avena (oat) extract, a ceramide and a dermatologically acceptable carrier. A method of treating symptoms of itch, redness, flaking, dryness, and / or roughness of the skin, scalp, and / or mucous membrane including the step of applying the topical skincare composition to at least a portion of a user's skin, scalp, and / or mucous membrane in need thereof.
Owner:THE PROCTER & GAMBLE COMPANY
Skin moisturizing lipstick
InactiveCN110151583AHas the effect of removing scarsIncrease vitalityCosmetic preparationsMake-upPlantago asiaticaOxalate
The invention discloses a skin moisturizing lipstick, which comprises a first ingredient, a second ingredient, a third ingredient and a fourth ingredient, wherein the first ingredient is prepared fromthe following ingredients (in percentage by mass): 55 to 65 of skin moisturizing agents, 2 to 7 of microcrystalline wax, 2 to 7 of phenyl trimethicone, 2 to 7 of polyethylene, 1.5 to 3.5 of bee wax,1.5 to 3.5 of simmondsiachinensis seed oil and 1.5 to 3.5 of silica dimethyl silylate; the second ingredient is prepared from the following ingredients (in percentage by mass): 10 to 15 of coloring agents; the third ingredient is prepared from the following ingredients (in percentage by mass): 2 to 5 of silica, 2 to 5 of water, 0.5 to 1.5 of plantago asiatica seed extracts and 0.5 to 1.5 of madecassic acid. The first ingredient, the second ingredient, the third ingredient and the fourth ingredient are combined to form the skin moisturizing lipstick according to the scientific and reasonable proportion; the skin moisturizing lipstick is mainly prepared from plant extracts, so that when the user uses the lipstick, no harm can be caused on the human body even when the lipstick enters the bodyof people.
Owner:广州市泫享生物科技有限公司
Damaged skin repairing gel and preparation process thereof
PendingCN112006938ARepair helpsIncrease surface areaCosmetic preparationsToilet preparationsGlycerolSkin repair
The invention relates to damaged skin repairing gel, which is composed of the following components in percentage by weight: a component A: water added to achieve a total volume of 100%; a component B:0.3 to 1.5 percent of an acrylic acid (acrylate) / C10-30 alkanol acrylate cross-linked polymer; a component C: 5-10 percent of water; a component D: 0.3 to 1.5 percent of p-hydroxyacetophenone, 0.1 to0.3 percent of allantoin, and 2 to 5 percent of glycerol; a component E: 2 to 5 percent of propylene glycol and 0.05 to 0.3 percent of sodium hyaluronate; a component F: 0.3 to 1.5 percent of triethanolamine, and 0.3 to 1.5 percent of water; a component G: 0.05 to 0.3 percent of dipotassium glycyrrhizinate and 1 to 3 percent of propylene glycol; a component H: 0.2 to 1 percent of 1, 2-hexanediol;a component I: 5-20 percent of calcium sodium phosphosilicate; a component J: 0.05 to 0.3 percent of asiaticoside, 0.05 to 0.3 percent of asiatic acid, 0.05 to 0.3 percent of madecassic acid and 2 to5 percent of water; a component K: 0.1 to 0.5 percent of silver oxide, 0.05 to 0.3 percent of inositol hexaphosphoric acid, 0.05 to 0.3 percent of pentasodium pentetate and 1 to 5 percent of ethanol;and a component L: 0.3 to 1.5 percent of glycerol glucoside. The invention also discloses a preparation method thereof. The gel has the advantages of being capable of repairing damaged skin and improving problematic skin and obvious in effect.
Owner:广州莱梧生物科技有限公司
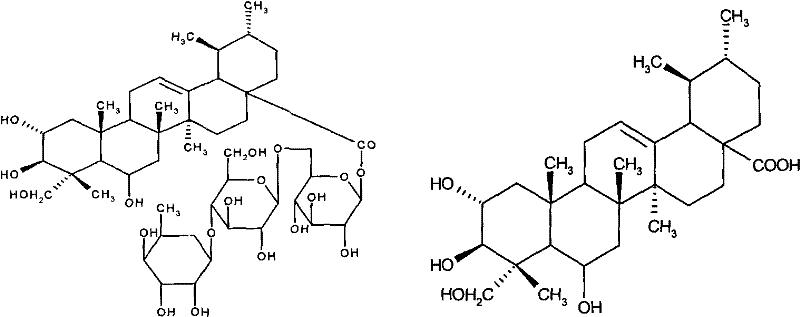
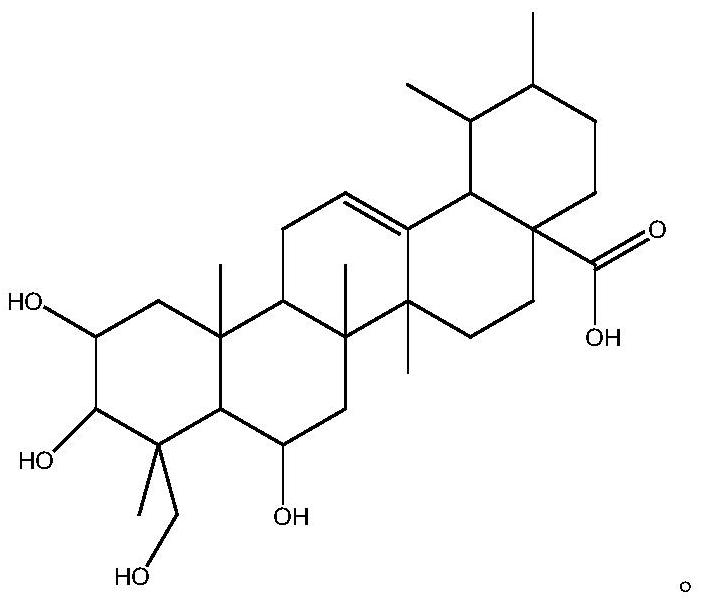
Method of preparing madecassic acid by hydrolyzing madecassoside
The invention discloses a method of preparing madecassic acid by hydrolyzing madecassoside. The method includes the steps of: 1) mixing madecassoside, lauryl sodium sulfate and NaOH, and adding water to dissolve the components to perform hydrolysis at normal temperature for 10-15 min; 2) regulating the pH of the hydrolysate to 2-5, allowing the hydrolysate to stand and filtering the hydrolysate, and collecting a precipitate; and 3) dissolving the precipitate in ethanol and filtering the solution, adding water to a filtrate to perform crystallization, filtering the liquid to obtain a crystal, and drying the crystal to obtain the madecassic acid. By means of the method, a glycosidic bond can be broken quickly just at normal temperature and under normal pressure. The hydrolysis process is mild and is complete. Configuration change of the madecassic acid is avoided in the invention.
Owner:广州深叶生物科技有限公司
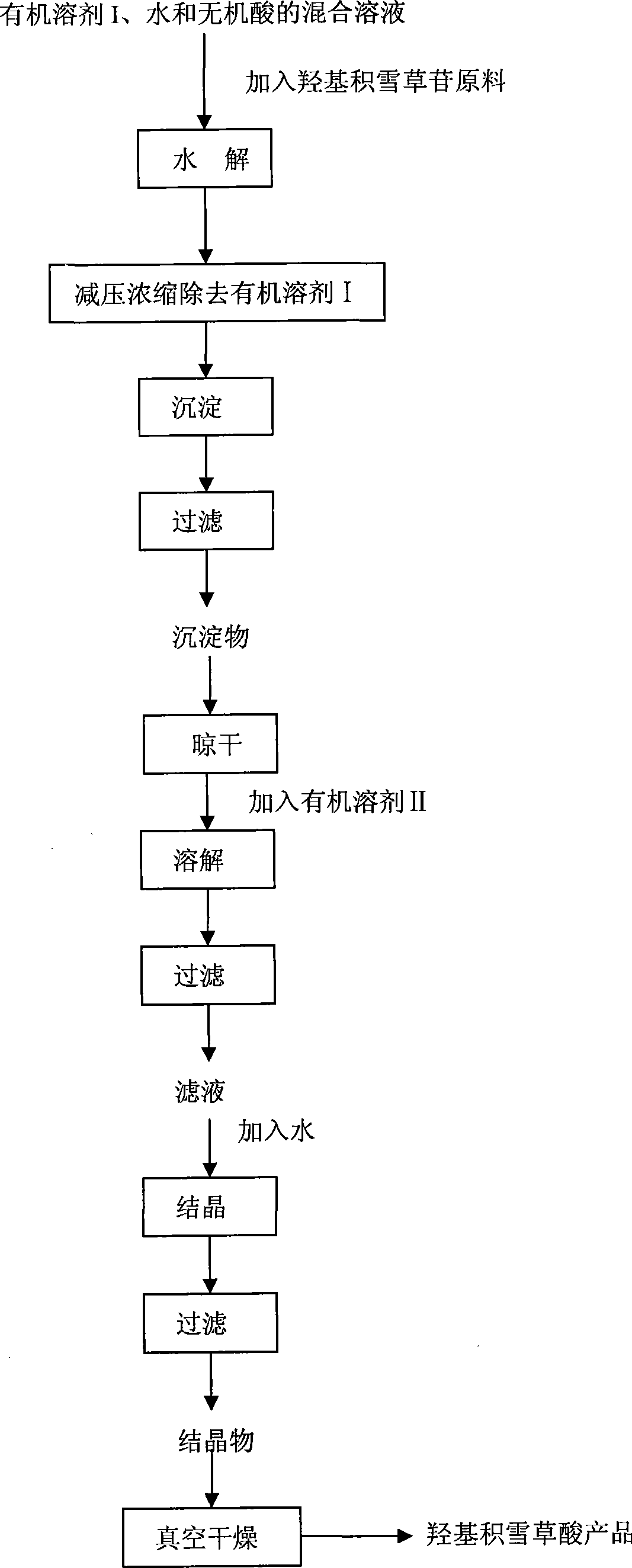
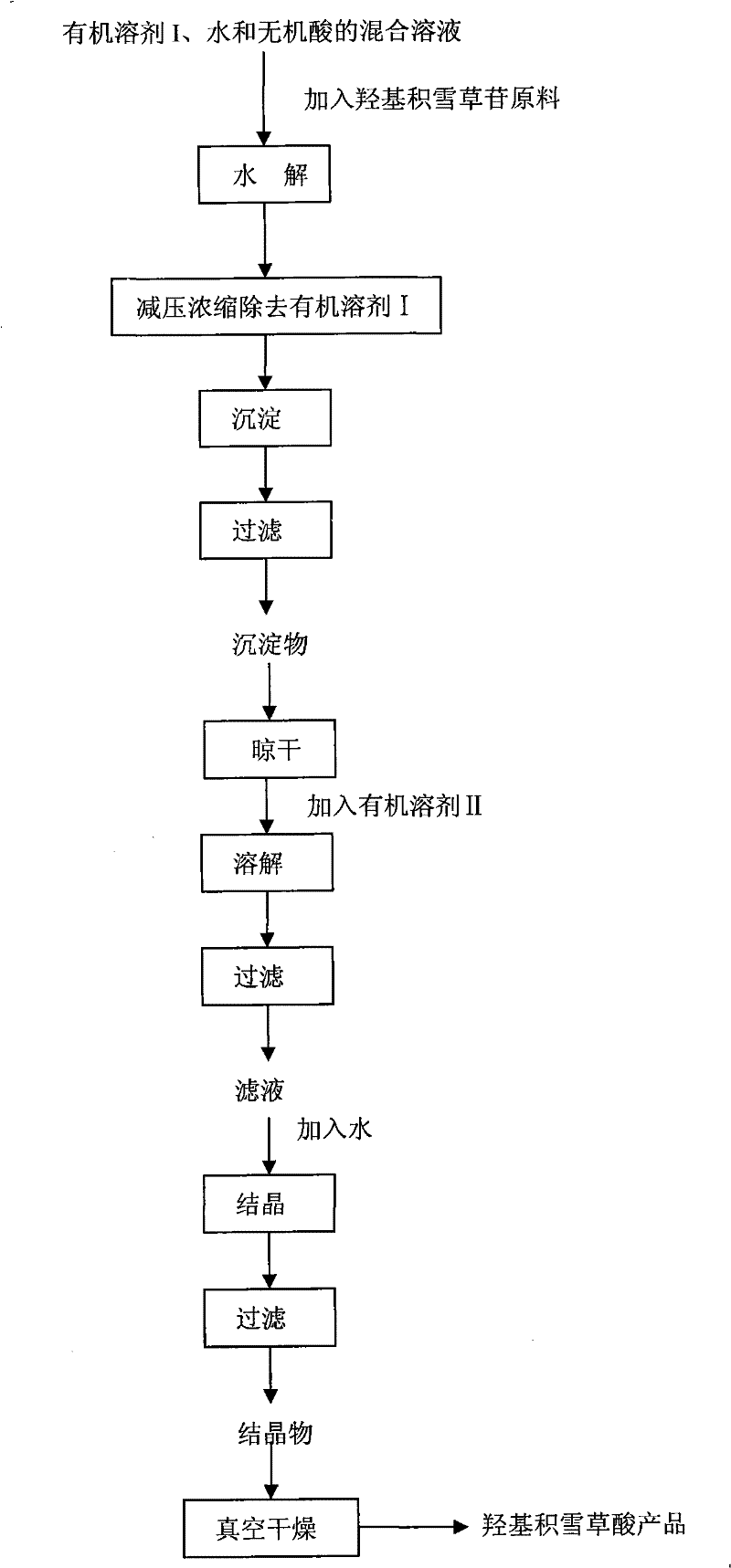
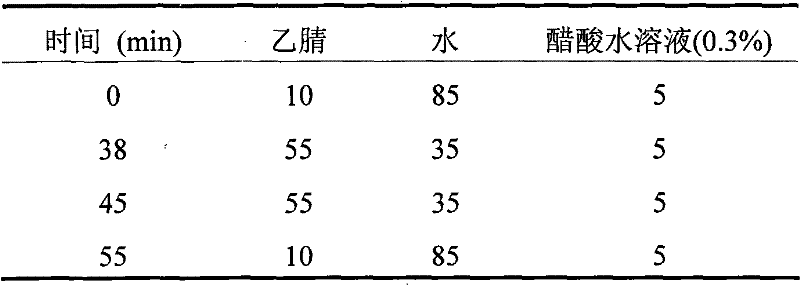
Method for preparing madecassic acid by hydroxyl asiatic centella glycosides acid hydrolysis
The invention discloses a method for preparing brahmic acid by acid hydrolysis of madecassoside, which comprises the following steps: 1) adding madecassoside raw material into a mixed solution of an organic solvent I, water and inorganic acid for hydrolysis at a temperature of between 45 and 80 DEG C for 1 to 24 hours; 2) concentrating the hydrolysate solution at reduced pressure to remove the organic solvent I, depositing and filtering the hydrolysate solution to obtain deposit; and 3) drying the deposit, adding the deposit into an organic solvent II for dissolution, filtering the solution, adding water into filtrate for crystallization, filtering the filtrate to obtain crystals, and drying the crystals in vacuum to obtain the brahmic acid product. The brahmic acid product has high yield and purity, and can be used for preparing bulk drug or derivatives of the brahmic acid; meanwhile, the method has simple technological process, small investment, and easy industrialization.
Owner:ZHEJIANG UNIV
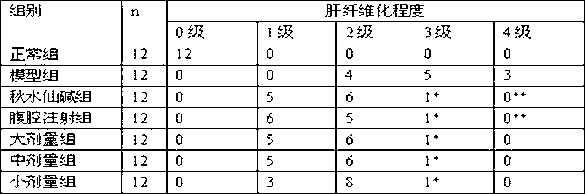
Use of madecassic acid of centella asiatica in preparing medicaments for treating liver fibrosis
InactiveCN102973579AReduce contentImprove liver functionOrganic active ingredientsDigestive systemSide effectMADECASSIC ACID
The present invention relates to the use of centella asiatica, and in particular relates to the use of monomer madecassic acid of the centella asiatica in preparing medicaments for treating liver fibrosis. The present invention also provides madecassic acid injection for the treatment of liver fibrosis and preparation method thereof. The present invention effectively broadens choices of liver fibrosis and cirrhosis patients, and compared with existing medicaments for treating liver fibrosis, the products of the present invention has the advantages of good efficacy, and little adverse side effect.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Method for separating and purifying Centella asiatica triterpene acid monoglucoside
The invention discloses a method for separating and purifying Centella asiatica triterpene acid monoglucoside by using crude Centella asiatica triterpene acid monoglucoside as the raw material. The method comprises the following steps: carrying out column chromatography on crude Centella asiatica triterpene acid monoglucoside, eluting using a mobile phase containing beta-cyclodextrin, collecting fractions respectively, and concentrating and drying to obtain asiatic acid monoglucoside, chebuloside II and madecassic acid monoglucoside. By adopting the technical scheme of the invention, pure Centella asiatica triterpene acid monoglucoside can be separated from crude Centella asiatica triterpene acid monoglucoside. In addition, the method is simple to operate, has lower cost and is suitable for large-scale production and applications.
Owner:SHANGHAI NORMAL UNIVERSITY
Nonapeptide-1 derivative as well as synthesis method and application thereof
ActiveCN113980098AGood transdermal permeabilityPromote absorptionCosmetic preparationsToilet preparationsWhitening AgentsMADECASSIC ACID
The invention discloses a nonapeptide-1 derivative as well as a synthesis method and application thereof, and belongs to the technical field of cosmetic peptides. The nonapeptide-1 and asiatic acid or madecassic acid are used as raw materials, the molar ratio of the nonapeptide-1 to the asiatic acid is 1: (2.0-4.0), or the molar ratio of the nonapeptide-1 to the madecassic acid is 1: (2.0-4.0), HOBt / DCC or DMAP / DCC is used as a condensing agent, condensation reaction is performed, and the nonapeptide-1 derivative is prepared. The nonapeptide-1 derivative disclosed by the invention has tyrosinase inhibition activity, antioxidant activity, anti-photoaging activity and anti-allergy activity, is good in transdermal permeability, and can be used for preparing a skin depigmenting agent, a whitening agent, a spot fading agent, an anti-aging agent and an anti-allergy agent.
Owner:浙江湃肽生物股份有限公司深圳分公司
Separation method of madecassic acid compound
ActiveCN108948123AExtended service lifeLow priceOrganic compounds purification/separation/stabilisationSteroidsCelluloseMADECASSIC ACID
The invention discloses a separation method of a madecassic acid compound. According to the separation method, separation is carried out by adopting a supercritical fluid chromatograph, and a cellulose chiral column is adopted as a chromatographic column. The separation effect of the method is good, the purity of a separated product is high, the separation speed is high, meanwhile, the service life of the chromatographic column is long, a solvent is low in cost, is free of pollution, and can be recycled, the separation efficiency is greatly improved, and the production cost is reduced.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Inhalation solution preparation containing Centella asiatica active ingredients and preparation method of inhalation solution preparation
PendingCN111374941AImprove stabilityClear efficacyDispersion deliveryInorganic non-active ingredientsMADECASSIC ACIDPreservative
The invention belongs to the field of pharmaceutics, and particularly relates to an inhalation solution preparation containing Centella asiatica active ingredients and a preparation method of the inhalation solution preparation. The solution preparation includes 1 [mu]g / mL-30 mg / mL of Centella asiatica active ingredients, an isotonic agent and a solvent, wherein the Centella asiatica active ingredients are one or more selected from Centella asiatica total asiaticosides, asiaticoside, madecassoside, asiaticoside B, asiatic acid, pharmaceutical salts of asiatic acid, madecassic acid, pharmaceutical salts of madecassic acid, asiatic acid B and pharmaceutical salts of asiatic acid B. The solution preparation has clear pharmaceutical effects and low content of impurities, the active ingredientsof Centella asiatica have good stability during long-term storage, and the content of main ingredients reaches 98% or above; the inhalation solution preparation has clear pharmaceutical effects, highstability, reliable quality, high safety and long storage time, and moreover, no preservatives are added to the solution preparation, so that the safety factor of the solution preparation is higher;and the solution preparation has suitable taste, and the taste of the active ingredients of Centella asiatica is covered fully.
Owner:INCREASEPHARM HENGQIN INST CO LTD
Method for preparing total asiatic acid, asiatic acid and madecassic acid from asiatic pennywort herb and use of prepared product
InactiveCN101991624BSimple processLow costOrganic active ingredientsDiabetes mellitusAsiatic pennywort
The invention relates to a method for preparing total asiatic acid, asiatic acid and madecassic acid from asiatic pennywort herb and use of a prepared product. The method comprises the following steps of: preparing asiaticoside from coarse powder of the asiatic pennywort herb; treating the asiaticoside by using alkali liquor, acidifying, extracting by using n-butyl alcohol, and recrystallizing by using alcohol to prepare the total asiatic acid; and performing chromatography on the total asiatic acid to obtain the asiatic acid and the madecassic acid. Meanwhile, the total asiatic acid, the asiatic acid and the madecassic acid can be used for reducing postmeal glucose and preventing and treating diabetes. The method has the characteristics that the process is simple, the cost is low, and the method is suitable for promotion and large-scale production.
Owner:SHANGHAI NORMAL UNIVERSITY
Madecassic acid solid lipid nanoparticle gel
PendingCN111920760AAnti-inflammatoryAnti-irritantOrganic active ingredientsAntipyreticIrritationSkin repair
The invention discloses a madecassic acid solid lipid nanoparticle gel. The madecassic acid solid lipid nanoparticle gel comprises madecassic acid solid lipid nanoparticles and blank gel, wherein theweight part ratio of the madecassic acid solid lipid nanoparticles to the blank gel is 25: 19, and the madecassic acid solid lipid nanoparticle gel has the effects of resisting inflammation and irritation, repairing true skin, inhibiting collagen synthesis, repairing surface skin, improving the keratinization function, resisting aging, stimulating skin cells and the like; through skin administration, the damage to the liver is avoided, the percutaneous penetration amount and bioavailability of the medicine are improved, and the purpose of sustained and controlled release targeted administration is achieved.
Owner:CHANGSHA MEDICAL UNIV
Method for extracting madecassic acid from Centella asiatica
The invention discloses a method for extracting madecassic acid from herba centellae. The method includes the following steps of firstly, smashing herba centellae, conducting reflux extraction through an ethyl alcohol solution with volume concentration of 60-70 v%, filtering an extraction solution, recovering solvent from filtrate, adding water at 40-50 DEG C for dissolving and filtering, and dissolving filter residues in an ethyl alcohol solution with volume concentration of 50-60 v% to obtain a crude extraction solution; secondly, adding rare-earth salts to the crude extraction solution to be mixed and evenly stirred to obtain mixed liquid; thirdly, applying the mixed liquid to an X-5 type macroporous resin column, conducting water washing till being colorless, conducting eluting through ethyl alcohol with volume concentration of 20-30 v%, conducting thin layer chromatography tracking and detecting, collecting ethyl alcohol eluent containing asiatic acid, conducting eluting through ethyl alcohol with volume concentration of 40-50 %, conducting thin layer chromatography tracking and detecting, collecting ethyl alcohol eluent containing madecassic acid, recovering ethyl alcohol, and conducting drying to obtain madecassic acid. The extracted madecassic acid is high in purity and high in yield.
Owner:桂林益天成生物科技有限公司
A method for preparing madecassic acid by hydrolyzing madecassic acid
The invention discloses a method for preparing madecassic acid by hydrolyzing madecasinic acid. The steps of the method are as follows: 1) Add madecassoside raw material into the mixed solution of organic solvent I, water and inorganic acid, and hydrolyze at 45-80° C. for 1-24 hours; 2) Concentrate the hydrolyzate under reduced pressure to remove organic solvent I , filtered after precipitation to obtain a precipitate; 3) After the precipitate was dried, an organic solvent II was added to dissolve and filter, and the filtrate was added with water to crystallize, and the crystal was obtained after filtration, and the crystal was vacuum-dried to obtain a product of madecassic acid. The product yield and purity of the madecassic acid of the invention are high, and the product can be used as a raw material medicine or the preparation of madecassic acid derivatives; meanwhile, the method has a simple process, requires less investment, and is easy to realize industrialization.
Owner:ZHEJIANG UNIV
Application of madecassic acid in preparation of medicine for preventing or treating ulcerative colitis
PendingCN112438981AGood treatment effectHigh activityOrganic active ingredientsDigestive systemSide effectUlcerative colitis
The invention discloses application of madecassic acid, or a salt thereof, or a complex thereof in preparation of a medicine for preventing or treating ulcerative colitis, and belongs to the field ofmedicines. Animal experiments show that the madecassic acid has a remarkable prevention and treatment effect on ulcerative colitis. The madecassic acid has an exact effect of resisting ulcerative colitis, is low in side effect, and has a wide medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for separating and purifying Centella asiatica triterpene acid monoglucoside
The invention discloses a method for separating and purifying Centella asiatica triterpene acid monoglucoside by using crude Centella asiatica triterpene acid monoglucoside as the raw material. The method comprises the following steps: carrying out column chromatography on crude Centella asiatica triterpene acid monoglucoside, eluting using a mobile phase containing beta-cyclodextrin, collecting fractions respectively, and concentrating and drying to obtain asiatic acid monoglucoside, chebuloside II and madecassic acid monoglucoside. By adopting the technical scheme of the invention, pure Centella asiatica triterpene acid monoglucoside can be separated from crude Centella asiatica triterpene acid monoglucoside. In addition, the method is simple to operate, has lower cost and is suitable for large-scale production and applications.
Owner:SHANGHAI NORMAL UNIVERSITY
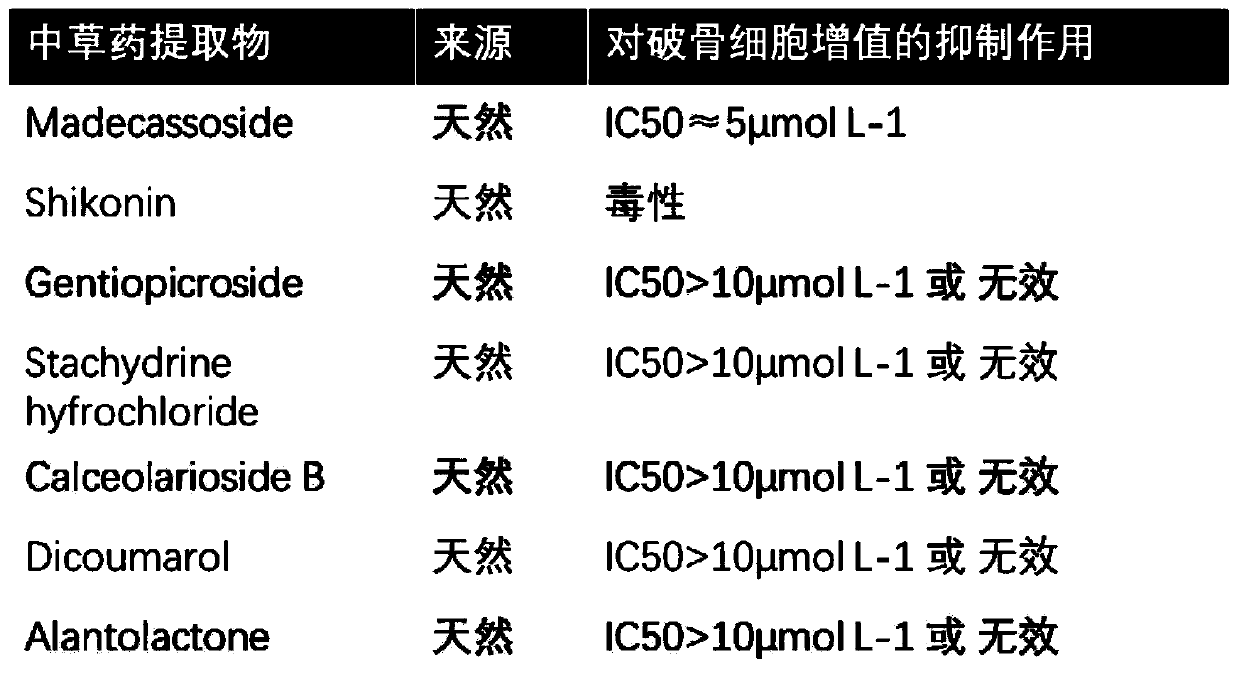
Application of signal pathway signal inhibitor or protein synthesis inhibitor or madecassic acid in preparation of drug for treating osteoporosis
InactiveCN110215517ASuppress generationSatisfied with the curative effectOrganic active ingredientsSkeletal disorderTherapeutic effectIsrapafant
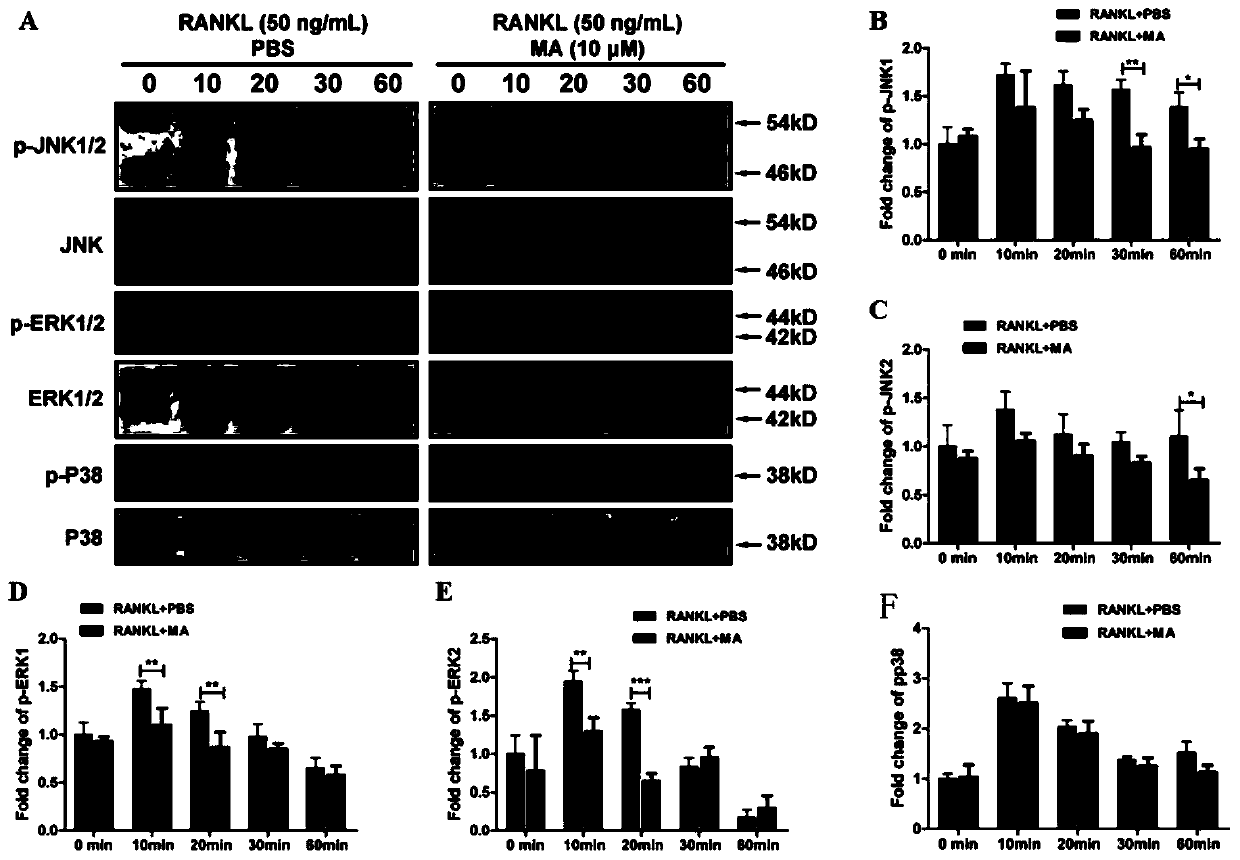
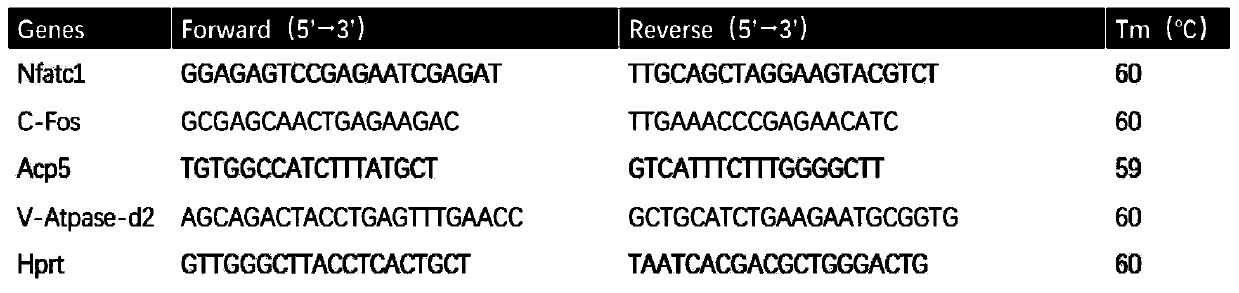
An application of a signal pathway signal inhibitor or a protein synthesis inhibitor or a madecassic acid in preparation of a drug for treating osteoporosis is disclosed. The madecassic acid inhibitsan expression of key protein-NFATc1 required for effect performance of osteoclasts through NF-[kappa]B, calcium / calmodulin-dependent kinase and MAPK three signal transduction pathways, and RANKL-induced osteoclast generation is ultimately inhibited. The application first describes and demonstrates that the madecassic acid has a satisfactory therapeutic effect on osteoporosis caused by estrogen deficiency, suggests that a natural compound madecassic acid extracted from Chinese herbal medicine has good application prospects in prevention and treatment of the osteoporosis, and provides a new option and a drug development idea for the treatment of the osteoporosis.
Owner:WENZHOU MEDICAL UNIV
Treatment of sebaceous gland disorders
PendingUS20220151954A1Improve efficiencyHighly effective against sebaceous glandCosmetic preparationsHydroxy compound active ingredientsDiseaseBacillus acnes
The present invention provides a topical formulation for use in a method of treating or preventing a sebaceous gland disorder selected from acne, seborrhea, rosacea, perioral dermatitis, corticosteroid-induced acneiform lesions and oily skin and / or for use in a method of inhibiting growth of riopionibacterium acnes, said method comprising topically administrating a formulation comprising (i) cannabidiol and (ii) triterpenes selected from asiaticoside, madecassoside, asiatic acid, madecassic acid and combinations thereof.The invention further relates to a topical formulation comprising:cannabidiol;triterpenes selected from asiaticoside, madecassoside, asiatic acid, madecassic acid and combinations thereof;flavonolignans selected silibinin, isosilibinin, silicristin, silidianin and combinations thereof, the concentrations of these flavonolignans being calculated as silibinin.
Owner:ECHO PHARM BV (NL)
A nonapeptide-1 derivative and its synthesis method and application
ActiveCN113980098BGood transdermal permeabilityPromote absorptionCosmetic preparationsToilet preparationsWhitening AgentsPreparing skin
Owner:浙江湃肽生物股份有限公司深圳分公司
Treatment of inflammatory skin conditions
PendingUS20220151953A1Improve efficiencyCosmetic preparationsHydroxy compound active ingredientsFlavonolignanCannabidiol
The present invention provides a topical formulation for use in a method of treating or preventing inflammatory skin conditions, said method comprising topically administrating a formulation comprising (i) cannabidiol and (ii) flavonolignans selected from silibinin, isosilibinin, silicristin, silidianin and combinations thereof.The invention further relates to a topical formulation comprising:cannabidiol;flavonolignans selected silibinin, isosilibinin, silicristin, silidianin and combinations thereof, the concentrations of these flavonolignans being calculated as silibinin;triterpenes selected from asiaticoside, madecassoside, asiatic acid, madecassic acid and combinations thereof.
Owner:ECHO PHARM BV (NL)
Method for extracting madecassic acid
The invention discloses a method for extracting madecassic acid. The method comprises the following steps: 1) smashing centella asiatica, refluxing and extracting with ethanol solution, filtering extract, recycling a solvent from filtrate, adding water at the temperature of 40-50 DEG C, dissolving, filtering, and dissolving filter residue in 50-60v% ethanol solution, so that crude extract is obtained; 2) purifying the crude extract with an XAD-9 macroporous resin column, eluting with acid liquor, and mixing effluent and pickling solution; and 3) regulating pH value of mixed solution to be neutral, filtering, purifying filtrate with an XAD-3 macroporous resin column, eluting with ethanol with the volume concentration of 30-40%, carrying out thin layer chromatography tracking detection, andcollecting ethanol eluate containing an asiatic acid ingredient; then eluting with ethanol with the volume concentration of 55-65%, carrying out thin layer chromatography tracking detection, collecting ethanol eluate containing madecassic acid, recycling ethanol from madecassic acid eluate, and drying, so that the madecassic acid is obtained. The extracted madecassic acid is high in purity, and yield is high.
Owner:无锡戴可思生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com