Inhalation solution preparation containing Centella asiatica active ingredients and preparation method of inhalation solution preparation
An active ingredient, the technology of Centella asiatica, applied in the field of pharmacy, can solve the problems of low bioavailability and poor therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 asiaticoside inhalation solution preparation
[0029] prescription:
[0030] Active ingredient: asiaticoside (purchased from the market, the content of asiaticoside, madecassoside and asiaticoside B is not less than 80wt%) 130mg;
[0031] Isotonic agent: sodium chloride (appropriate amount, adjust osmotic pressure (osmolality) to 295mOsmol / kg);
[0032] pH adjuster: 1wt% HCl aqueous solution (adjust pH to 5);
[0033] Water for injection: 1000mL.
[0034] Take the prescription amount of asiaticosides and water for injection, mix them evenly at room temperature, add sodium chloride to adjust the osmotic pressure, add a pH regulator to adjust the pH, and constant volume to obtain the asiaticosides solution for inhalation; The total glucoside solution is filled into the ampoule bottle, and the asiaticoside solution preparation for inhalation of the present invention is obtained.
Embodiment 2
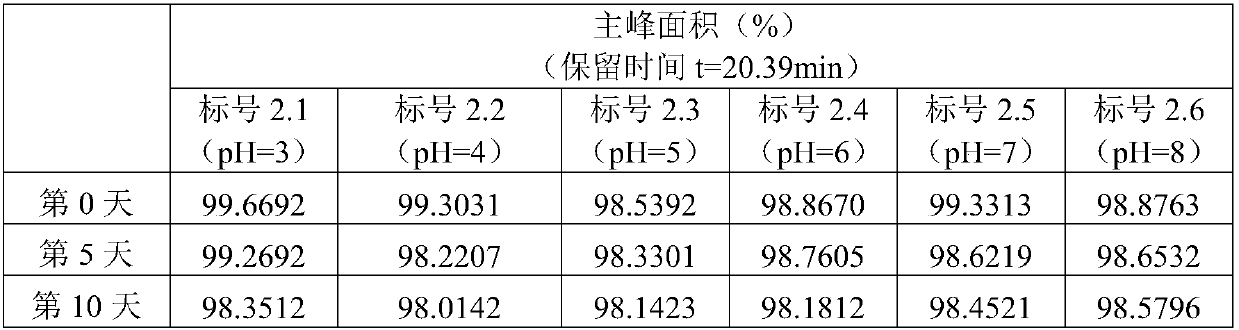
[0035]Example 2 Preparations of asiaticoside inhalation solutions with different pH values
[0036] The physical properties of the inhalation solution will affect the compliance of the patient. For example, the pH value may cause unnecessary mucosal irritation to the patient, and it will also affect the stability of the inhalation solution itself, affecting the quality of the inhalation solution, and ultimately causing unnecessary harm to the patient. risk of use. In order to overcome the influence of pH value, the inventors investigated the stability of asiaticoside inhalation solution formulations under different pH conditions.
[0037] prescription:
[0038] Active ingredient: Asiaticoside 150mg;
[0039] Isotonic agent: sodium chloride (appropriate amount, adjust osmotic pressure (osmolality) to 298mOsmol / kg);
[0040] pH adjuster: 1wt% HCl aqueous solution (adjust pH to 3, 4, 5, 6); 0.2mol / L NaOH aqueous solution (adjust pH to 8);
[0041] Water for injection: 1000mL....
Embodiment 3
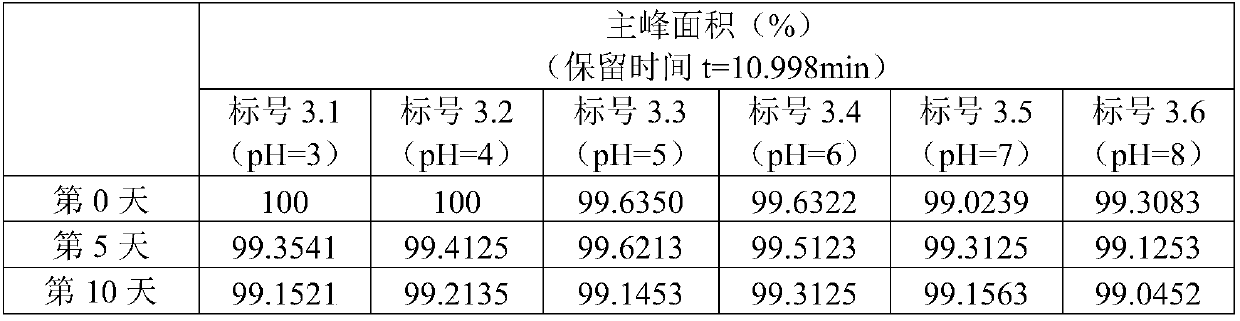
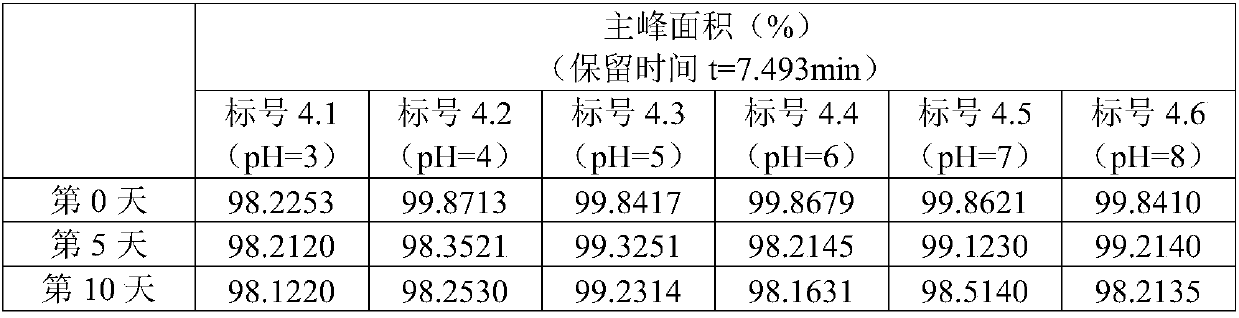
[0047] Example 3 Formulations of Madecassoside Inhalation Solution with Different pH Values
[0048] In order to overcome the influence of pH value, the inventors investigated the stability of madecassoside inhalation solution formulations under different pH conditions.
[0049] prescription:
[0050] Active ingredient: Madecassoside 130mg;
[0051] Isotonic agent: sodium chloride (appropriate amount, adjust osmotic pressure (osmolality) to 290-310mOsmol / kg);
[0052] pH adjuster: 1wt% HCl aqueous solution (adjust pH to 3, 4, 5, 6); 0.2mol / L NaOH aqueous solution (adjust pH to 8);
[0053] Water for injection: 1000mL.
[0054] Take the prescribed amount of madecassoside and add them to 6 volumetric flasks, add water for injection to the above volumetric flasks, mix well at room temperature, add sodium chloride to adjust the osmotic pressure, and add pH regulators to adjust the pH value 3, 4, 5, 6, 7 (no pH regulator), 8, constant volume to obtain the madecassoside solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com