A nonapeptide-1 derivative and its synthesis method and application
A synthesis method and derivative technology, applied in the field of cosmetic peptides, can solve the problem of nonapeptide-1 derivatives, etc., and achieve the effects of inhibiting tyrosinase activity, scavenging DPPH free radicals, and preventing skin aging.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
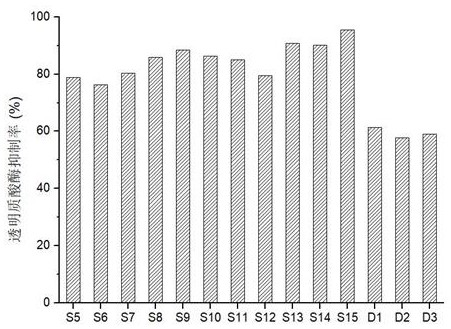
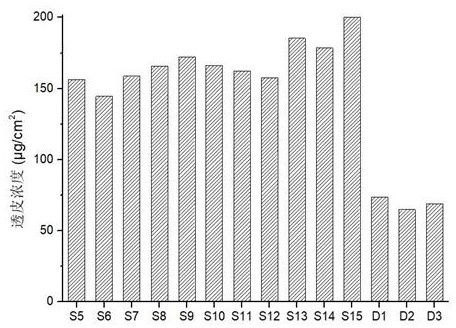
Examples
Embodiment 1
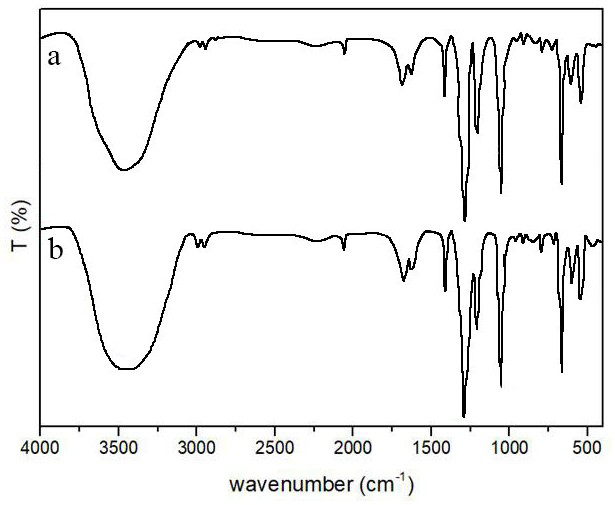
[0048] The synthetic method of nonapeptide-1-Aa derivative
[0049] Adding nonapeptide-1, asiatic acid, HOBt and DCC to CH 2 Cl 2 , stirred at room temperature for 24 h, after the reaction, the solvent was distilled off under reduced pressure, distilled water and ethyl acetate were added for extraction, the organic layer was washed with distilled water, hydrochloric acid solution, saturated sodium bicarbonate solution and saturated sodium chloride solution successively, and finally Dry over anhydrous sodium sulfate, concentrate under reduced pressure, and finally recrystallize from chloroform-methanol to obtain nonapeptide-1-Aa derivatives with a yield of 81.15%.
[0050] Wherein, the molar ratio of nonapeptide-1 and asiatic acid is 1:3.0, the molar ratio of asiatic acid, HOBt and DCC is 1:1.05:1.05, nonapeptide-1 and CH 2 Cl 2 The dosage ratio of nonapeptide-1 and ethyl acetate is 1 g: 6 mL; during the extraction process, the volume ratio of distilled water and ethyl aceta...
Embodiment 2
[0052] The synthetic method of nonapeptide-1-Aa derivative
[0053] The raw materials used in this example and the synthesis method are basically the same as those in Example 1, except that the used condensing agent HOBt / DCC is replaced by DMAP / DCC, and the molar ratio of Asiatic acid, DMAP and DCC is 1:0.2:1.05. The yield of nonapeptide-1-Aa derivative was 83.42%.
Embodiment 3
[0055] The synthetic method of nonapeptide-1-Ma derivative
[0056] The raw materials used in this example and the synthesis method are basically the same as those in Example 1, the difference being that the asiatic acid used is replaced by madecassic acid. The yield of nonapeptide-1-Ma derivative was 80.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com