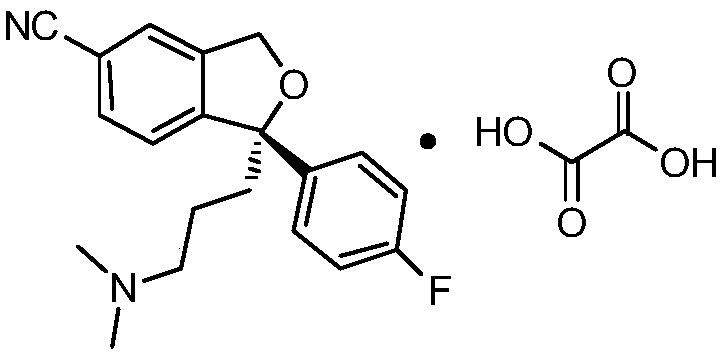
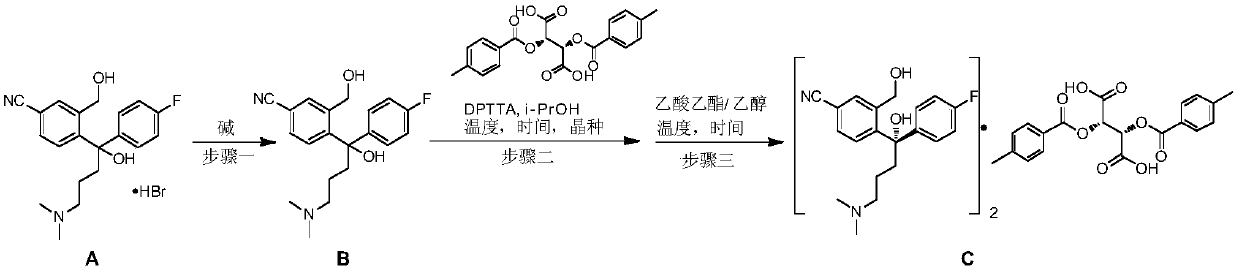
Preparation method of high-optical-purity escitalopram oxalate intermediate S-configuration diol
A technology for escitalopram oxalate and intermediates, which is applied in the field of preparation of high-purity escitalopram oxalate intermediates S-configuration diol, can solve the problem of poor reproducibility, yield of only 20.3%, There are no problems such as resolution effects, and the effects of high product yield, high optical purity, and high resolution efficiency are achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 (S)-(4-(4-dimethylamino-1-p-fluorophenyl-1-hydroxybutyl)-3-(hydroxymethyl)benzocyanide) D-(+)- Preparation of di-p-methylbenzoyl tartrate
[0026] (1) At room temperature, (RS)-4-(4-(dimethylaminopropyl)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-hydroxymethylbenzonitrile hydrogen Bromate (85.0g, 0.2mol) was added to 200ml of an aqueous solution containing 12.0g of NaOH, dichloromethane (200ml) was added, and stirred for 0.5 hour; the liquid was left to separate, and the aqueous phase was extracted twice with dichloromethane (50ml). Combine the organic phases, dry with anhydrous sodium sulfate, remove the solvent under reduced pressure, remove residual methylene chloride with isopropanol (20ml) to obtain an oily substance, add isopropanol (200ml) and heat to 70 ° C, after completely dissolving, Add D-(+)-di-p-methylbenzoyl tartaric acid (40.0g, 0.1mol), after completely dissolved, stop stirring, naturally cool down to 20-25°C, add a little seed crystal (1‰) at 60°C...
Embodiment 2
[0029] Example 2 (S)-(4-(4-dimethylamino-1-p-fluorophenyl-1-hydroxybutyl)-3-(hydroxymethyl)benzocyanide) D-(+)- Preparation of di-p-methylbenzoyl tartrate
[0030] (1) At room temperature, (RS)-4-(4-(dimethylaminopropyl)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-hydroxymethylbenzonitrile hydrogen Bromate (85.0 g, 0.2 mol) was added to 200 ml of an aqueous solution containing 12.0 g of NaOH, dichloromethane (200 ml) was added, and stirred for 0.5 hour; standing for liquid separation, the aqueous phase was extracted twice with dichloromethane (50 ml), Combine the organic phases, dry with anhydrous sodium sulfate, remove the solvent under reduced pressure, remove residual methylene chloride with isopropanol (20ml) to obtain an oily substance, add isopropanol (300ml) and heat to 70 ° C, after completely dissolving, Add D-(+)-di-p-methylbenzoyl tartaric acid (40.0g, 0.1mol), after completely dissolved, stop stirring, naturally cool down to 20-25°C, add a little seed crystal (1‰) at 60°...
Embodiment 3
[0033] Example 3 (S)-(4-(4-dimethylamino-1-p-fluorophenyl-1-hydroxybutyl)-3-(hydroxymethyl)benzocyanide) D-(+)- Preparation of di-p-methylbenzoyl tartrate
[0034] (1) At room temperature, (RS)-4-(4-(dimethylaminopropyl)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-hydroxymethylbenzonitrile hydrogen Bromate (85.0 g, 0.2 mol) was added to 200 ml of a solution containing 12.0 g of NaOH, dichloromethane (200 ml) was added, and stirred for 0.5 hours. Stand for liquid separation, extract the aqueous phase twice with dichloromethane (50ml), combine the organic phases, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and remove residual dichloromethane with isopropanol (20ml) to obtain an oily Add isopropanol (400ml) and heat to 70°C. After it is completely dissolved, add D-(+)-di-p-methylbenzoyl tartaric acid (40.0g, 0.1mol). After it is completely dissolved, stop stirring and let it cool down naturally. To 20-25°C, add a little seed crystal (1‰) at 60°C, stan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com