Patents
Literature
41 results about "Orodispersible tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
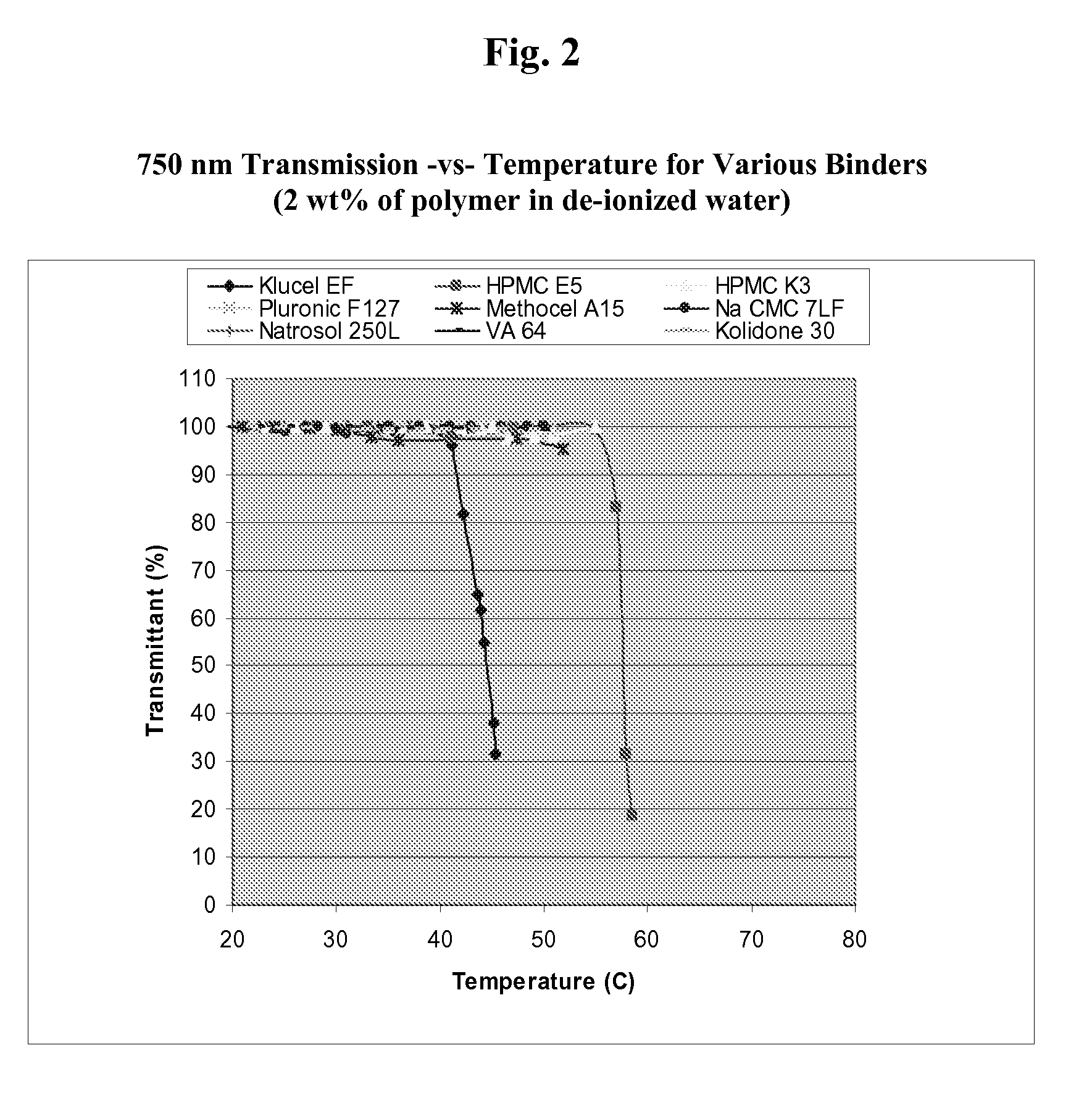
Orodispersible tablets were placed in basket sinker in the middle of the vessel with a distance of 6-8.5 cm. Even Narazaki et al. carried out the disintegration test with rotary-shaft method. The apparatus consisted of stainless steel wire gauze on which orodispersible tablets were placed and slightly immersed in medium.
Orodispersible tablets
ActiveUS20100297031A1Short disintegration timeGood mechanical resistanceBiocideNervous disorderCalcium silicateOrally disintegrating tablet
This invention relates to a an orally disintegrating tablet obtainable by direct compression of a dry powdered mixture, said mixture comprising up to 15% by weight of calcium silicate, at least 50% of a diluent, a disintegrant agent and an active ingredient. It also relates to a process for preparing the tablets by homogeneous blending the specific excipients in powder form and subsequent direct compression of the mixture. Said tablets disintegrate quickly in the cavity of the mouth, in particular in less than 15 seconds.
Owner:LAB LESVI SL
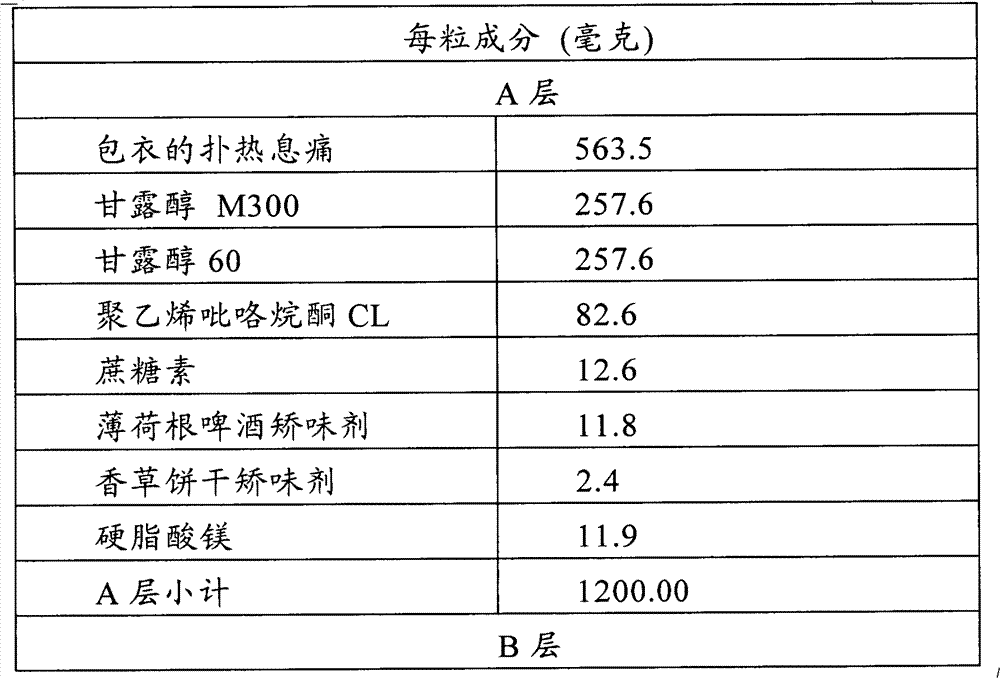
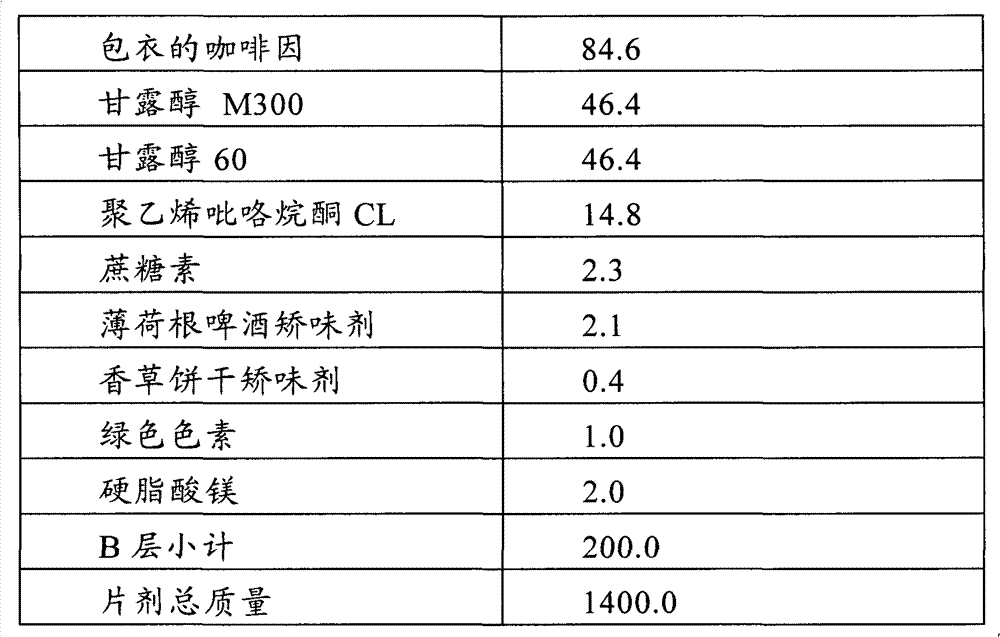
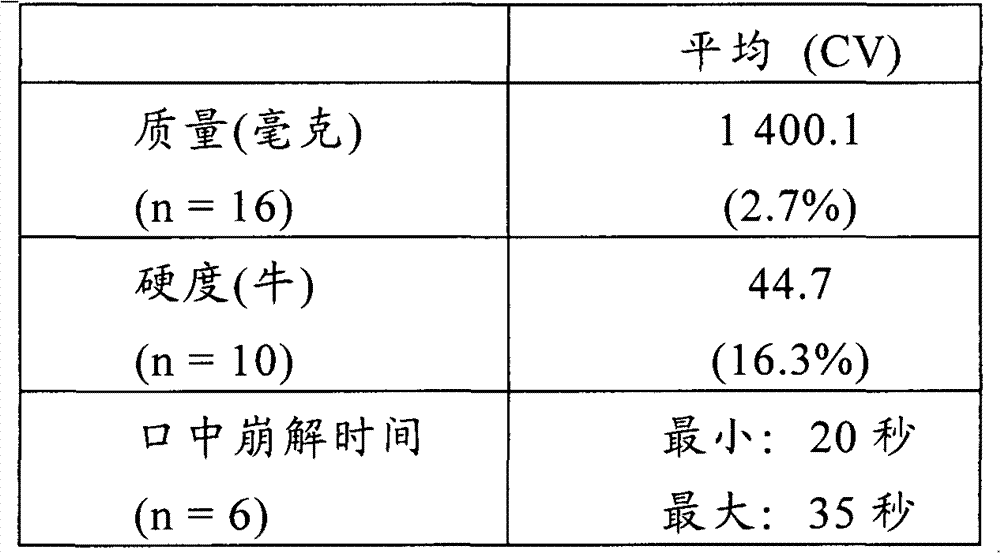
Multilayer orodispersible tablet
The present invention relates to a multilayer orodispersible tablet and to the process for preparing it.
Owner:ETHYPHARM SA
Taste-masked oral formulations of influenza antivirals
The present invention relates to taste-masked oral formulations of influenza antivirals. The taste-masked pharmaceutical formulations for oral administration comprise one or more influenza antivirals, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. Further, the taste-masked influenza antiviral formulations of the present invention are provided in the form of dispersible tablets, effervescent tablets, orally disintegrating tablets, chewable tablets, bite-dispersion tablets or the like, wherein the bitter taste of influenza antivirals is masked thereby providing palatable formulations.
Owner:RUBICON RES PTY LTD
Orally-dispersible multilayer tablet
The present invention relates to a multilayer orodispersible tablet and to the process for preparing it.
Owner:ETHYPHARM SA
Orodispersible tablets
InactiveUS20120077888A1Short disintegration timeHighly robustBiocideAntipyreticMANNITOL/SORBITOLActive agent
A directly compressed orodispersible tablet comprises 0.1 to 50% of a ungranulated active agent (w / w), 10 to 80% of a sugar-based direct compression base, and 10 to 80% of a microcrystalline cellulose (MCC) direct compression base, and has a hardness of at least 60N, and a disintegration time of less than 40 seconds. The sugar-based direct compression base is a DC sugar alcohol, especially direct compression mannitol, and the MCC base is a silicified MCC, especially a Prosolv. The active is a hydrophobic active, typically a high-dose active. Also disclosed is a method of producing an orodispersible tablet comprising the steps of directly compressing a mixture of components at a compression force of at least 5 k N to form the tablet, wherein the mixture of components comprises 0.1 to 50% of an active agent (w / w), 10 to 80% of a sugar-based direct compression base (w / w); and 10 to 80% of a microcrystalline cellulose (MCC) direct compression base (w / w).
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
Rapidly Disintegrating Dosage Form Comprising Magnesium Carbonate Heavy
InactiveUS20080166406A1Good dispersionDissolve fastBiocideInorganic non-active ingredientsOrodispersible tabletMoisture
A rapidly disintegrating dosage form containing magnesium carbonate heavy is described, which disintegrates upon contact with moisture. The dosage forms can be either dispersible or orodispersible tablets and can accommodate widely different active principles. The magnesium carbonate heavy is found to be an excellent dispersant under basic and neutral conditions, and gives the tablets a smooth mouth-feel.
Owner:ACTAVIS GRP PTC EHF
Orodispersible tablets containing fexofenadine
The present invention concerns orodispersible tablets, which are able to disintegrate in the buccal cavity upon contact with saliva by formation of an easy-to-swallow suspension, in less than 60 seconds, preferably in less than 40 seconds, containing fexofenadine in the form of coated granules, and a mixture of excipients comprising at least one disintegrating agent, a soluble diluent agent, a lubricant and optionally a swelling agent, a permeabilising agent, sweeteners, flavoring agents and colors; the process for obtaining such orodispersible tablets and the coated granules incorporated therein and the use of said orodispersible tablets in the treatment of seasonal allergic rhinitis.
Owner:ETHYPHARM SA
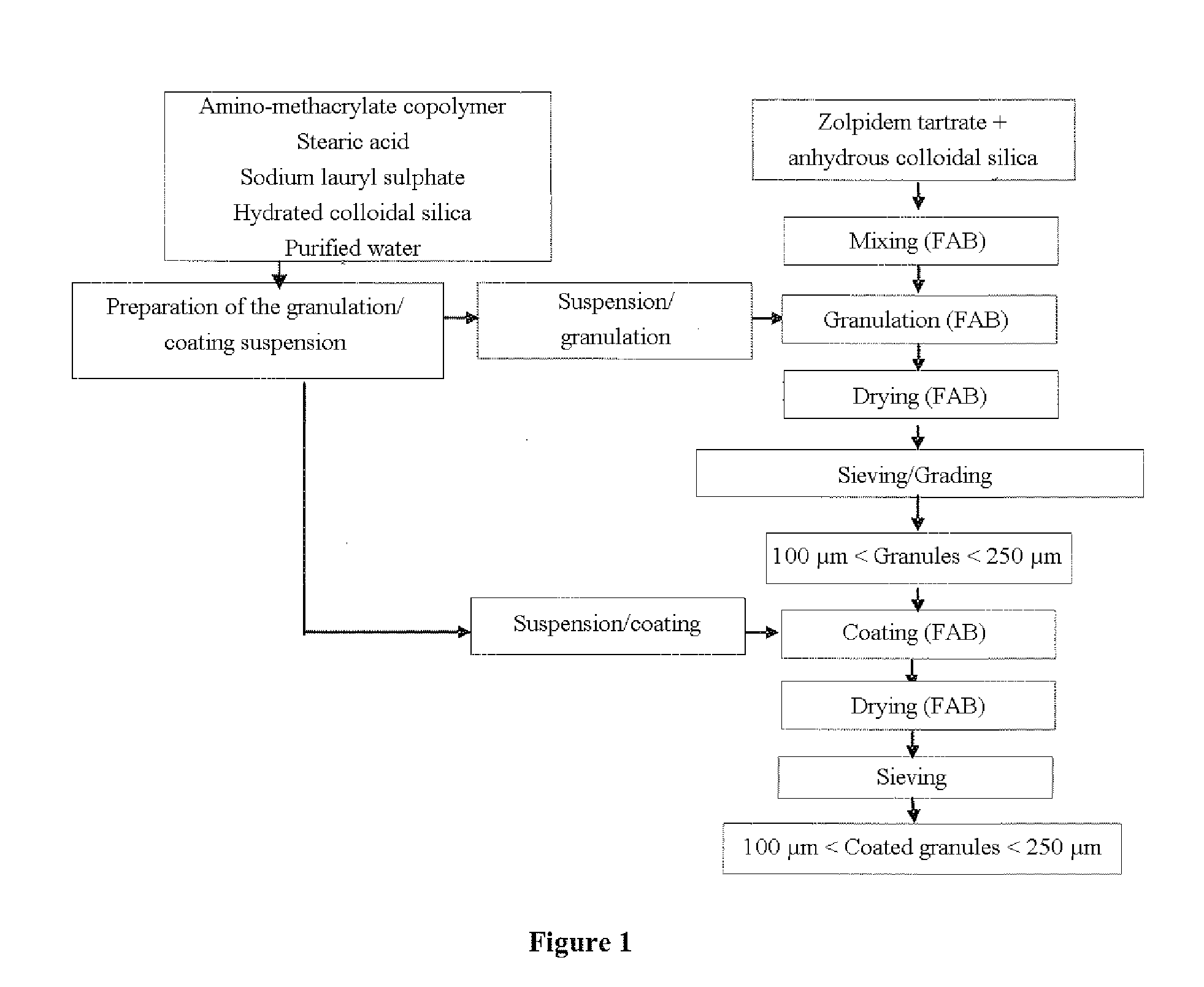
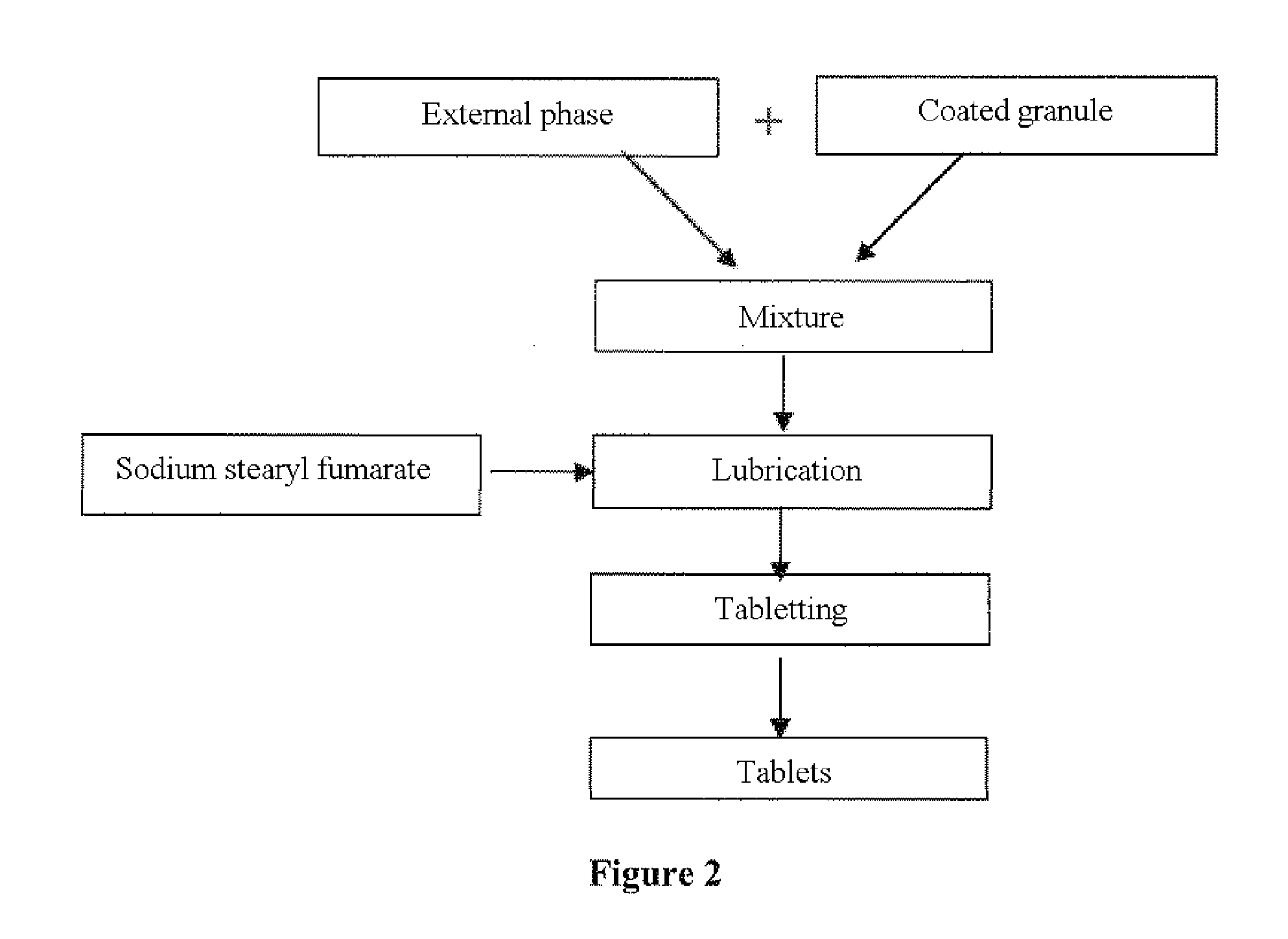
Zolpidem-based orodispersible pharmaceutical tablet
The present invention is directed to oral pharmaceutical forms for rapid disintegration which make it possible to prevent possible misuse of the zolpidem present therein. The present invention thus relates to a zolpidem-based orodispersible tablet formulation intended to prevent abuse of use of the tablet at the expense of a third party.
Owner:SANOFI SA
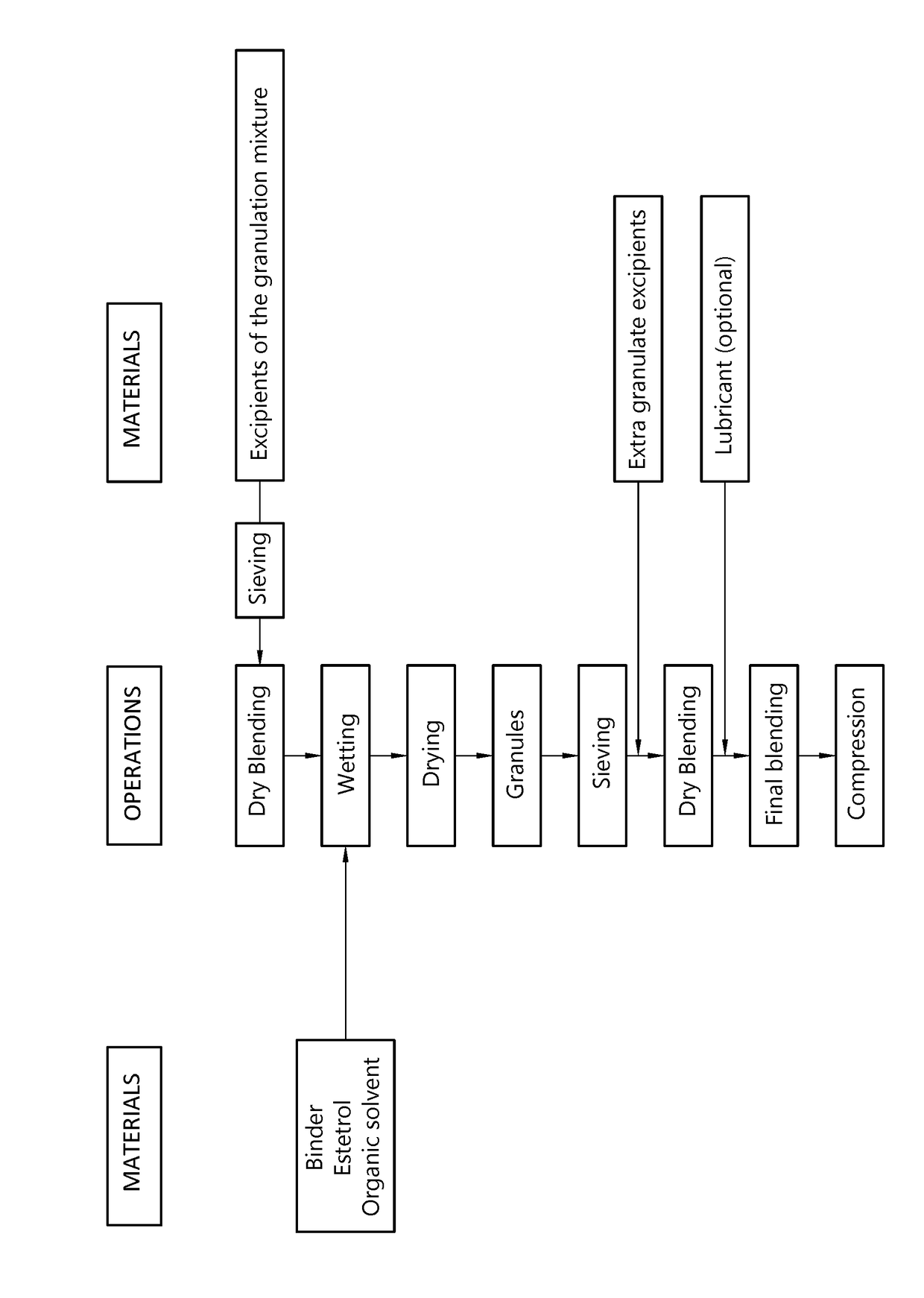
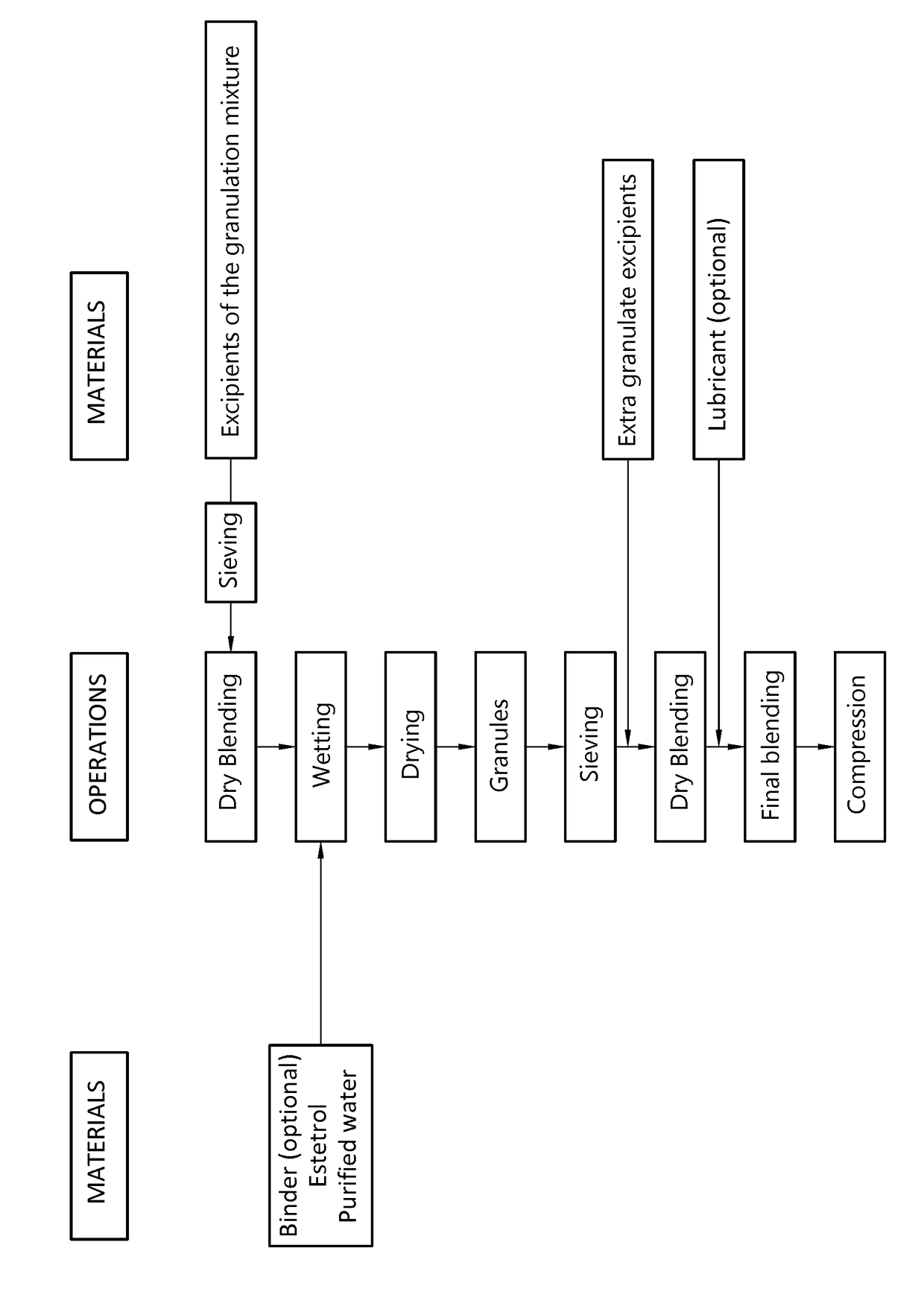
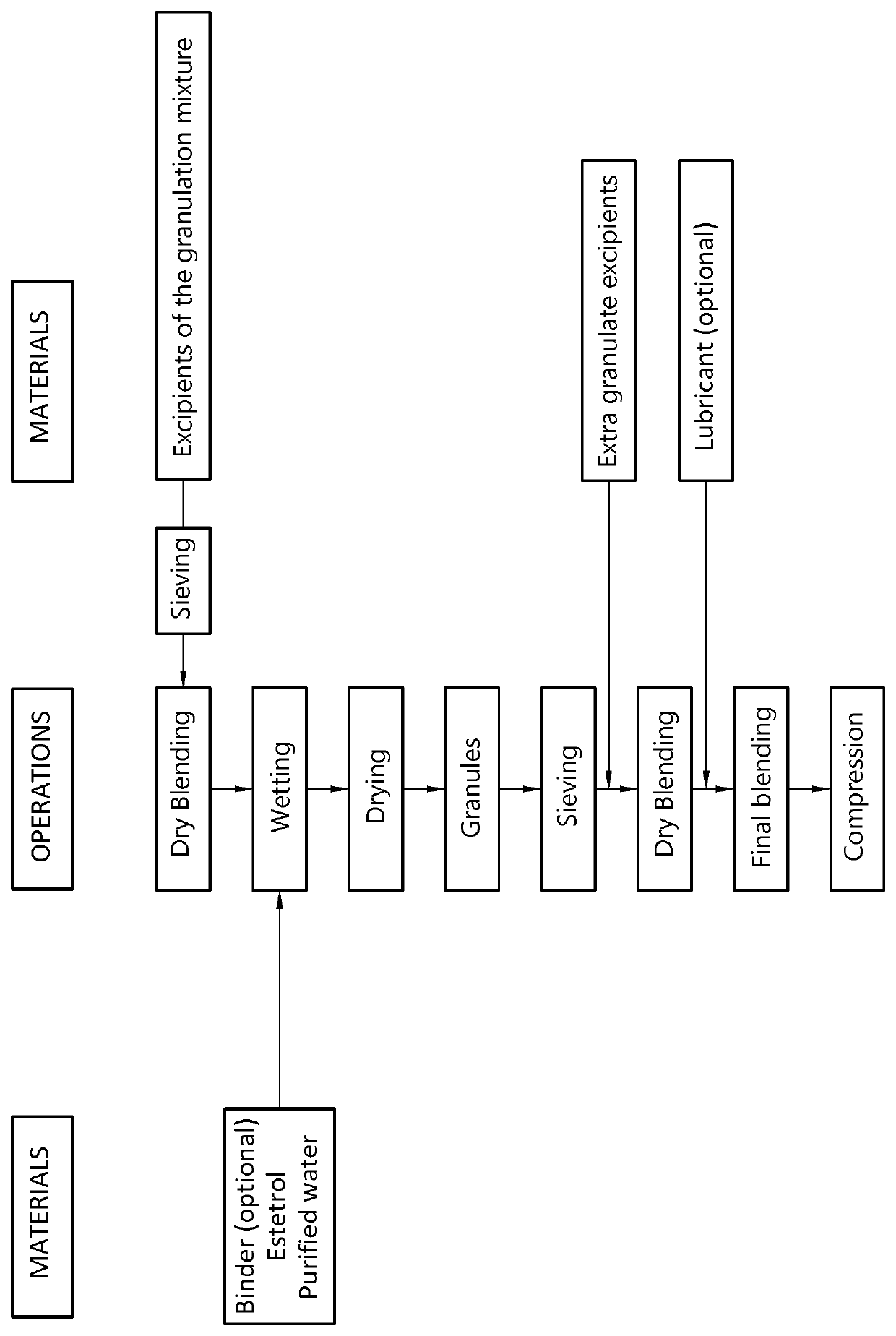
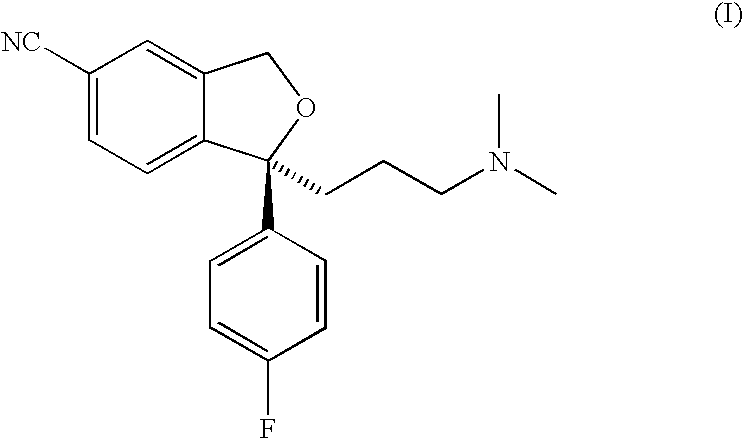
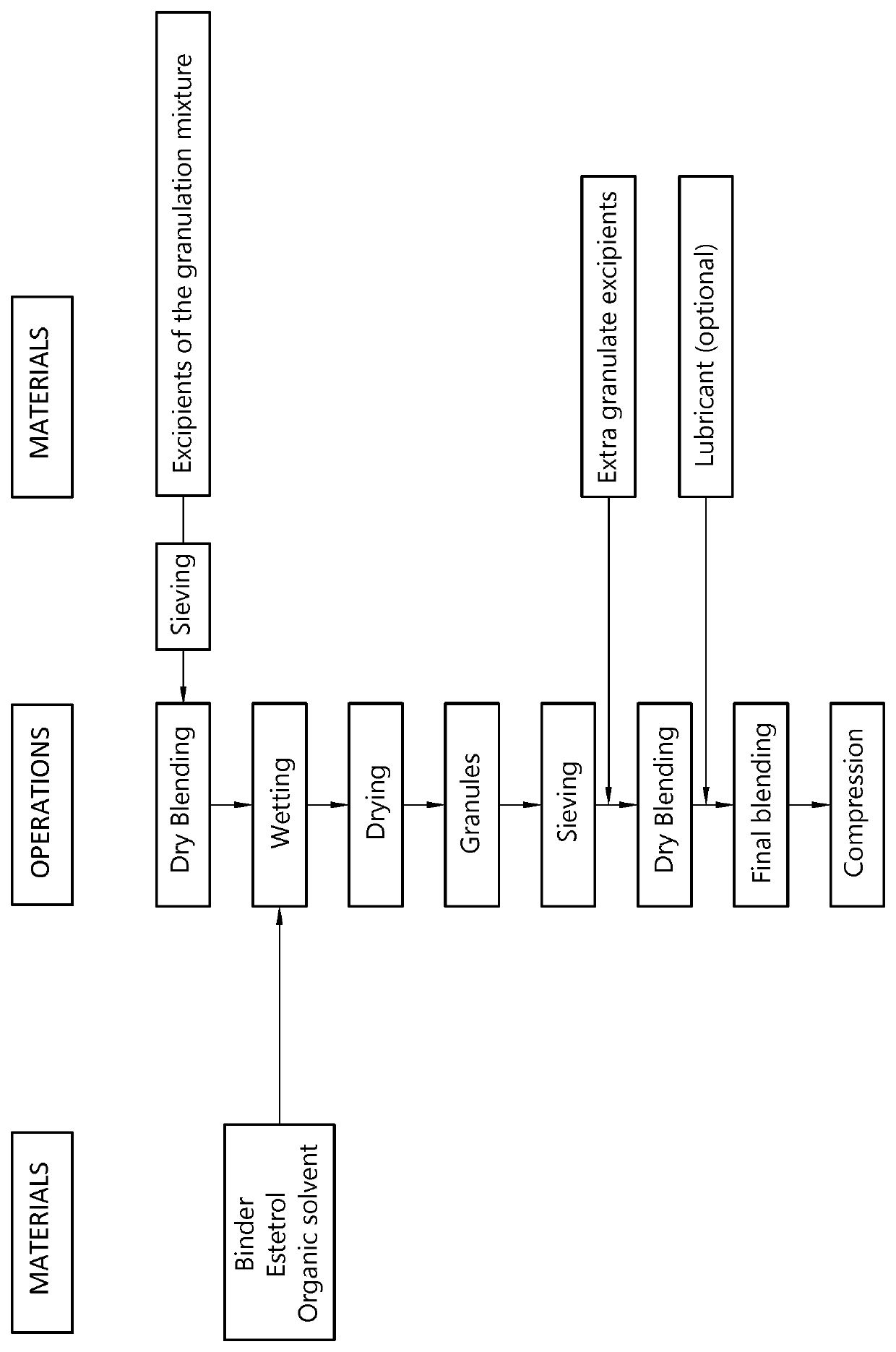
Orodispersible tablet containing estetrol
ActiveUS20180185271A1Easy to manufactureAvoids first-pass liver exposureOrganic active ingredientsPill deliveryOrganic solventSublabial administration
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit containing at least 100 μg of an estetrol component selected from estetrol, estetrol esters and combinations thereof; wherein the solid dosage unit can be obtained by a process that comprises: providing a loading liquid comprising organic solvent; estetrol component and optionally one or more other pharmaceutically acceptable ingredients; mixing 1 part by weight of the loading liquid with 0.5-20 parts by weight of carrier particles to produce wet particles; removing organic solvent from the wet particles to produce loaded particles; optionally mixing the loaded particles with one or more tabletting excipients; and forming the loaded particles or the mixture of the loaded particles and the one or more tabletting excipients into a solid dosage unit. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Oral dosage form
InactiveUS20170071988A1Achieve effectUnknown materialsUsing mechanical meansOrodispersible tabletDentistry
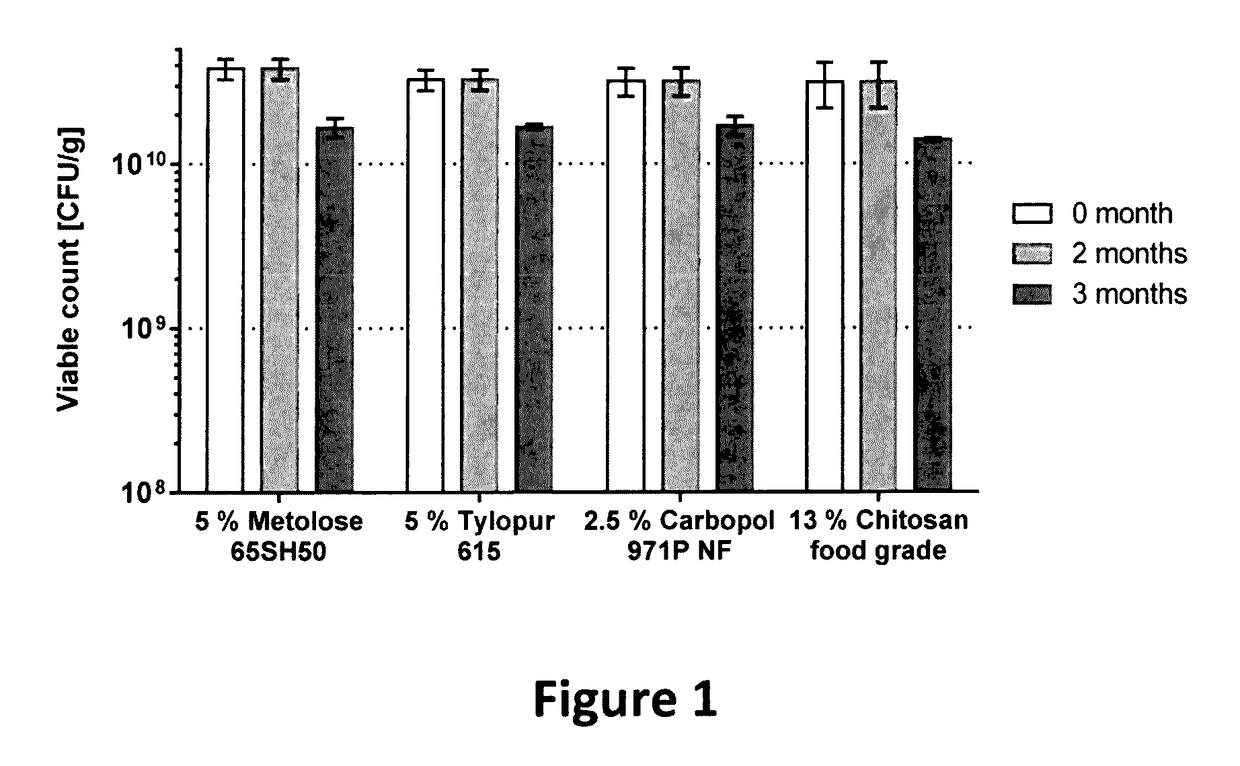
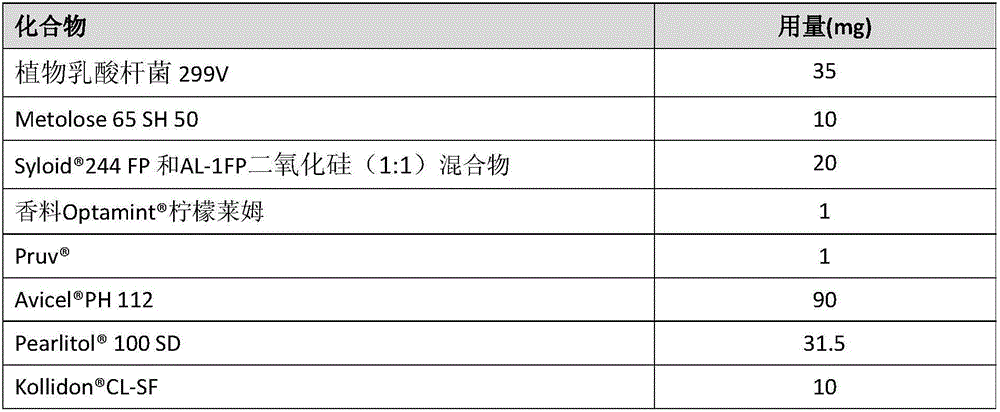
The present invention is in the field of delivering an active substance to the oral cavity and relates to mucoadhesive active composition and corresponding mucoadhesive dosage form, which can deliver an active substance within the oral cavity, especially an orodispersible tablet for delivering probiotic substance. The present invention also relates to a method of producing the said composition and a method of processing the composition into a mucoadhesive dosage form, especially an orodispersible tablet. The present invention moreover relates to a system and a method for testing the mucoadhesion of a dosage form.
Owner:SYMRISE GMBH & CO KG
Orodispersible tablet containing estetrol
ActiveUS20180153801A1Easy to manufactureAvoids first-pass liver exposureOrganic active ingredientsPill deliverySublabial administrationMedicine
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit containing at least 100 μg of an estetrol component selected from estetrol, estetrol esters and combinations thereof; wherein the solid dosage unit can be obtained by a process comprising: ⋅providing an aqueous liquid comprising water, estetrol component and optionally one or more other pharmaceutically acceptable ingredients; ⋅mixing 1 part by weight of the aqueous liquid with 0.5-20 parts by weight of the carrier particles to produce wet particles; ⋅removing water from the wet particles to produce loaded particles; ⋅optionally mixing the loaded particles with one or more tabletting excipients; and ⋅forming the loaded particles or the mixture of loaded particles and the one or more tabletting excipients into a solid dosage unit. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Fast onset orodispersable tablets
ActiveUS8343978B2Reduce brittlenessImprove bioavailabilityOrganic active ingredientsBiocideActive agentOrodispersible tablet
The present invention provides orodispersable tablets and methods of using the same. The tablets and methods are useful, for example, for reducing first pass metabolism of orally administered active agents, enhancing bioavailability of active agents, and / or reducing the time it takes for an active agent to achieve maximal effect in a subject. The tablets, when taken orally, disintegrate or dissolve rapidly such that active agent included in the tablets is absorbed in the buccal cavity. The invention further provides methods of manufacturing any of the tablets disclosed herein and containers that include any of the tablets disclosed herein.
Owner:SINOTHERAPEUTICS
Orodispersible tablet containing estetrol
ActiveUS10888518B2Easy to manufactureQuick effectOrganic active ingredientsPill deliverySublabial administrationPharmaceutical medicine
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit containing at least 100 μg of an estetrol component selected from estetrol, estetrol esters and combinations thereof; wherein the solid dosage unit can be obtained by a process comprising: ⋅providing an aqueous liquid comprising water, estetrol component and optionally one or more other pharmaceutically acceptable ingredients; ⋅mixing 1 part by weight of the aqueous liquid with 0.5-20 parts by weight of the carrier particles to produce wet particles; ⋅removing water from the wet particles to produce loaded particles; ⋅optionally mixing the loaded particles with one or more tabletting excipients; and ⋅forming the loaded particles or the mixture of loaded particles and the one or more tabletting excipients into a solid dosage unit. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Rapidly disintegrating dosage form comprising magnesium carbonate heavy
A rapidly disintegrating dosage form containing magnesium carbonate heavy is described, which disintegrates upon contact with moisture. The dosage forms can be either dispersible or orodispersible tablets and can accommodate widely different active principles. The magnesium carbonate heavy is found to be an excellent dispersant under basic and neutral conditions, and gives the tablets a smooth mouth-feel.
Owner:ACTAVIS GRP PTC EHF
Multilayer orodispersible tablet
Owner:ETHYPHARM SA
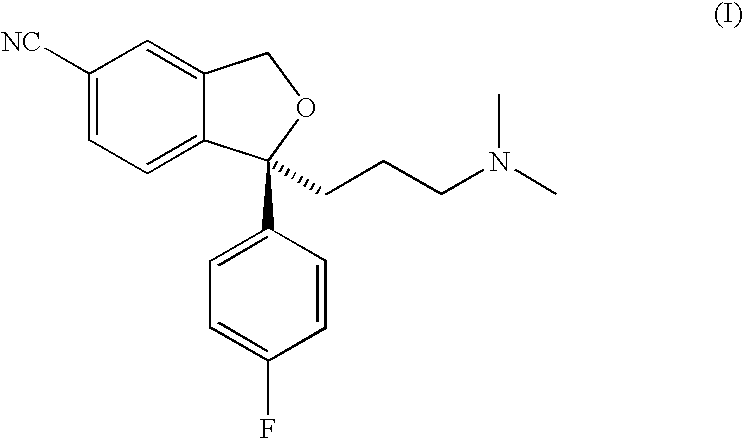
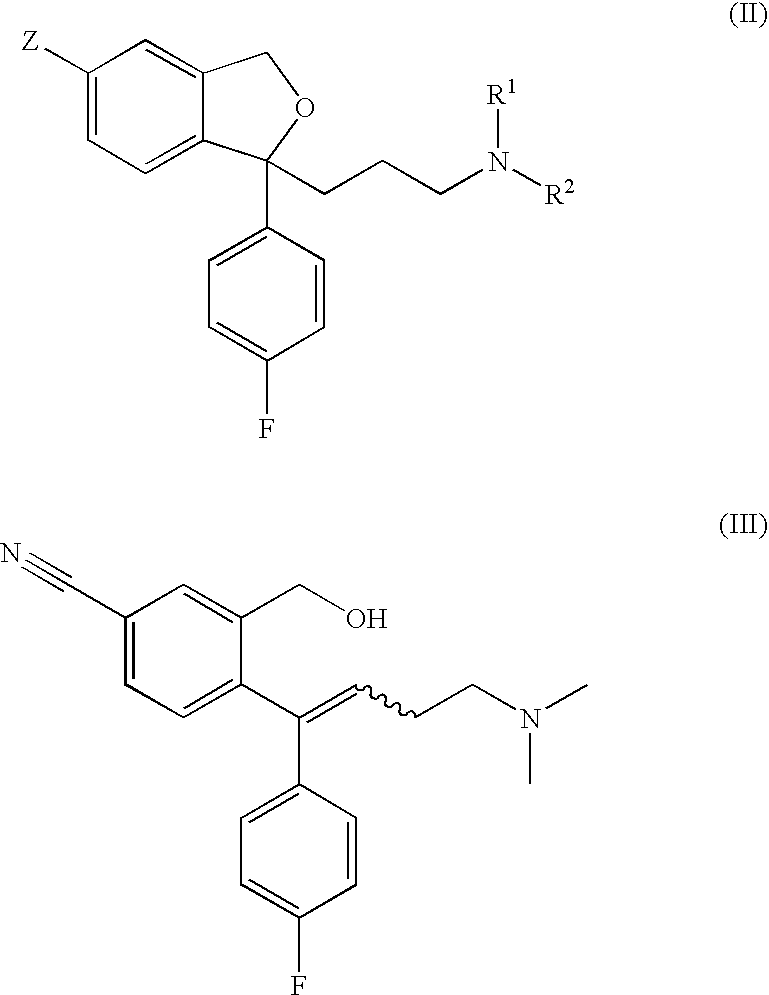
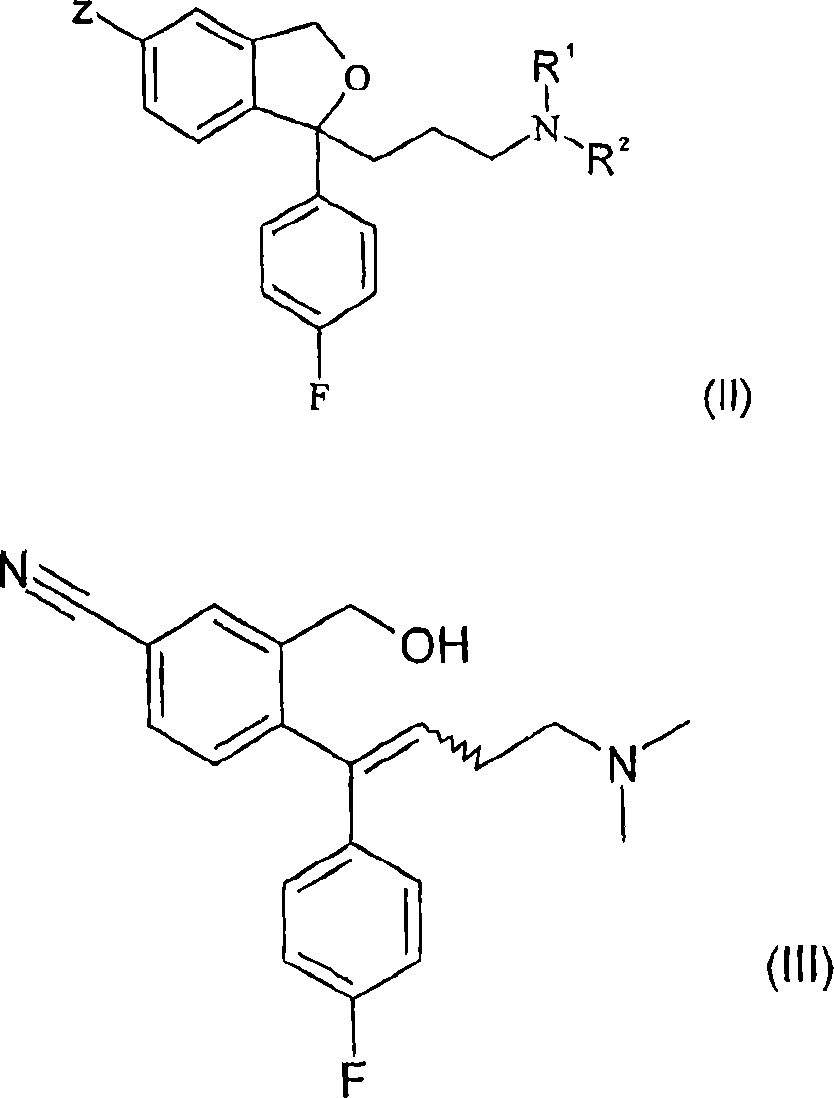
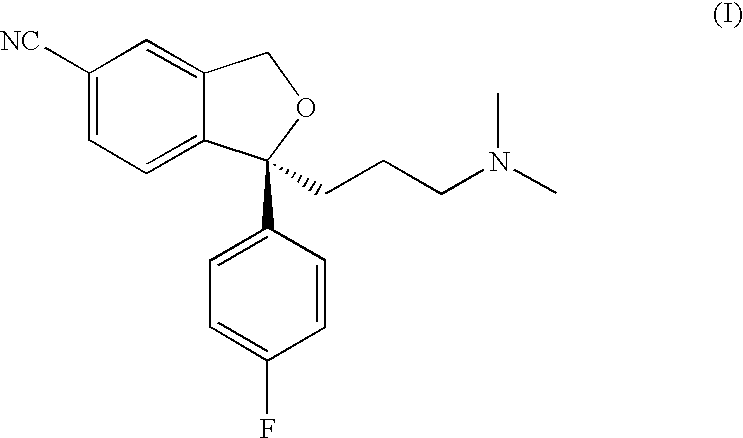
Crystalline base of escitalopram and orodispersible tablets comprising escitalopram base
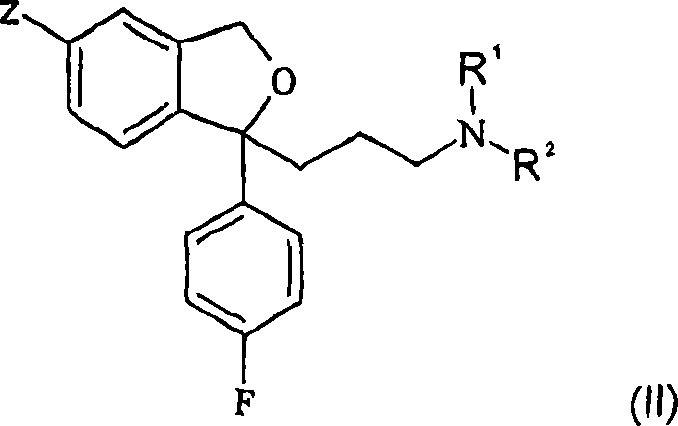
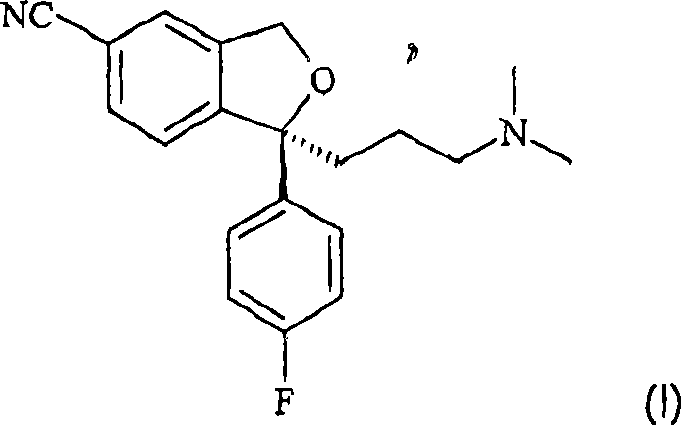
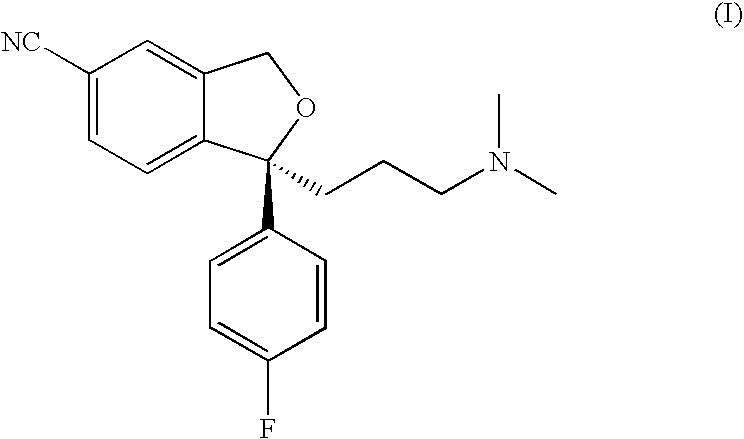
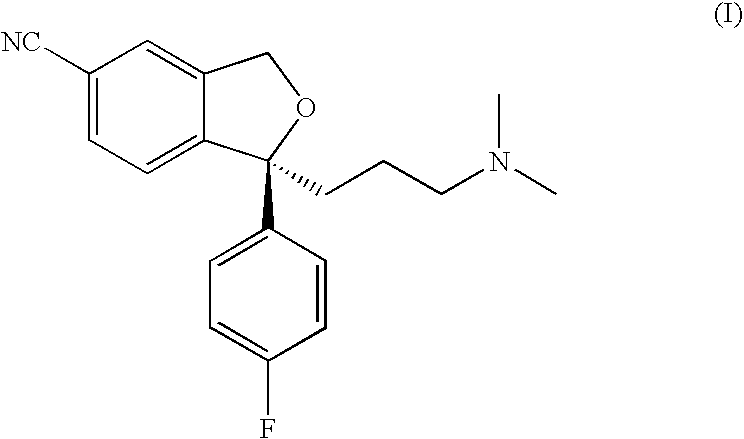
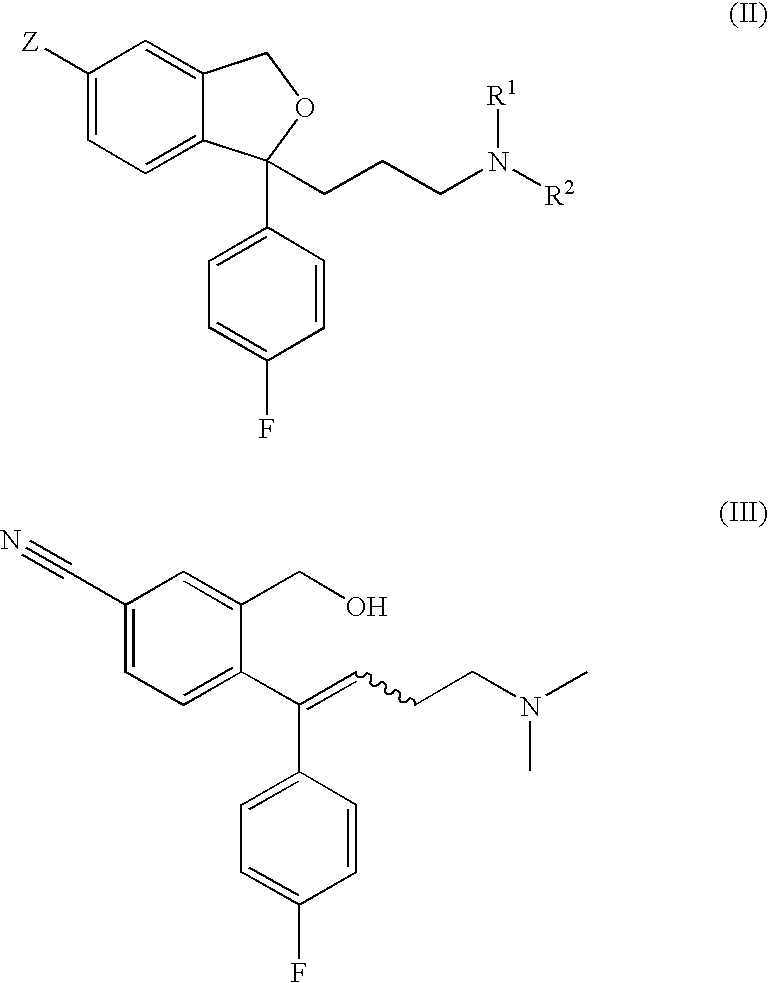
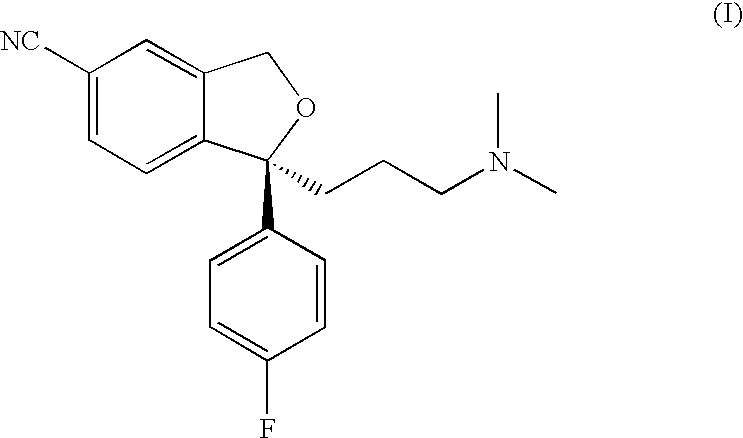
The present invention relates to the crystalline base of the well known antidepressant drug escitalopram, S-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile, formulations of said base, a process for the preparation of purified salts of escitalopram, such as the oxalate, using the base, the salts obtained by said process and formulations containing such salts, and a process for the preparation of purified escitalopram free base or salts of escitalopram, such as the oxalate, using the hydrobromide, the salts obtained by said process and formulations containing such salts. Finally the present invention relates to an orodispersible tablet having a hardness of at least 22 N and an oral-disintegration time of less than 120 s and comprising an active pharmaceutical ingredient adsorbed onto a water soluble filler wherein the active pharmaceutical ingredient has a melting point in the range of 40-100° C., as well as a method for making such an orodispersible tablet.
Owner:H LUNDBECK AS
Orodispersible tablet containing Estetrol
ActiveUS10894014B2Easy to manufactureQuick effectOrganic active ingredientsPill deliveryOrganic solventSublabial administration
The invention provides an orodispersible solid pharmaceutical dosage unit having a weight between 30 and 1,000 mg, said dosage unit containing at least 100 μg of an estetrol component selected from estetrol, estetrol esters and combinations thereof; wherein the solid dosage unit can be obtained by a process that comprises: providing a loading liquid comprising organic solvent; estetrol component and optionally one or more other pharmaceutically acceptable ingredients; mixing 1 part by weight of the loading liquid with 0.5-20 parts by weight of carrier particles to produce wet particles; removing organic solvent from the wet particles to produce loaded particles; optionally mixing the loaded particles with one or more tabletting excipients; and forming the loaded particles or the mixture of the loaded particles and the one or more tabletting excipients into a solid dosage unit. The solid dosage unit is easy to manufacture and perfectly suited for sublingual, buccal or sublabial administration.
Owner:ESTETRA SRL
Crystalline base of escitalopram and orodispersible tablets comprising escitalopram base
InactiveCN101189220AOrganic active ingredientsOrganic compounds purification/separation/stabilisationOxalateHydrobromide
The present invention relates to the crystalline base of the well known antidepressant drug escitalopram, S-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile, formulations of said base, a process for the preparation of purified salts of escitalopram, such as the oxalate, using the base, the salts obtained by said process and formulations containing such salts, and a process for the preparation of purified escitalopram free base or salts of escitalopram, such as the oxalate, using the hydrobromide, the salts obtained by said process and formulations containing such salts. Finally the present invention relates to an orodispersible tablet having a hardness of at least 22 N and an oral-disintegration time of less than 120 s and comprising an active pharmaceutical ingredient adsorbed onto a water soluble filler wherein the active pharmaceutical ingredient has a melting point in the range of 40-100 DEG C, as well as a method for making such an orodispersible tablet.
Owner:H LUNDBECK AS
Orodispersible domperidone tablets
InactiveCN101394850BHigh hardnessImprove featuresOrganic active ingredientsDigestive systemMANNITOL/SORBITOLMedicine
The present invention relates to an orodispersible tablet comprised of, by weight: a maximum of 15% of a low-dose, therapeutically active substance; from 55% to 70% of mannitol of a particle size between 30 μm and 300 μm; at least 2% of maltodextrin; from 3.5% to 8% of croscarmellose sodium; from 10% to 20% of microcrystalline cellulose; from 0.5% to 1.5% of magnesium stearate; and from 1% to 5% of flavoring (s) and sweetener (s).
Owner:PIERRE FABRE MEDICAMENT SAS
Formulations for systemic buccal delivery comprising s-adenosylmethionine, their preparation and use
Formulations for systemic buccal delivery, in particular chewing gums, chewable tablets, orodispersible tablets, oromucosal preparations comprising sulpho-adenosyl-L-methionine (SAMe) which allows the absorption of the active principle through the oral mucosa are described.
Owner:SSTREETCARENTINOLI GIORGIO +1
Granulate for the formulation of orodispersible tablets
InactiveUS20100260854A1High porosityAvoid high pressurePowder deliveryBiocideMANNITOL/SORBITOLMedicine
This invention relates to a granulate comprising mannitol and sorbitol in a weight ratio of between 70:30 and 97:3. This invention also relates to the use of the said granulate in the preparation of orodispersible tablets, to the orodispersible tablets obtained with the said granulate and to a process of production for obtaining the said granulate.
Owner:E PHARM TRENTO SPA
Crystalline base of escitalopram and orodispersible tablets comprising escitalopram base
The present invention relates to the crystalline base of the well known antidepressant drug escitalopram, S-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzo-furancarbonitrile, formulations of said base, a process for the preparation of purified salts of escitalopram, such as the oxalate, using the base, the salts obtained by said process and formulations containing such salts, and a process for the preparation of purified escitalopram free base or salts of escitalopram, such as the oxalate, using the hydrobromide, the salts obtained by said process and formulations containing such salts. Finally the present invention relates to an orodispersible tablet having a hardness of at least 22 N and an oral-disintegration time of less than 120 s and comprising an active pharmaceutical ingredient adsorbed onto a water soluble filler wherein the active pharmaceutical ingredient has a melting point in the range of 40-100° C., as well as a method for making such an orodispersible tablet.
Owner:H LUNDBECK AS
Oral dosage form
ActiveCN106822007AMucoadhesive simpleLow costUnknown materialsUsing mechanical meansBiochemistryOrodispersible tablet
Owner:SYMRISE GMBH & CO KG
Orally-dispersible multilayer tablet
The present invention relates to a multilayer orodispersible tablet and to the process for preparing it.
Owner:ETHYPHARM SA
Orodispersible tablets of erythritol and isomalt
Erythritol is granulated together with at least 10% w / w isomalt. Prior and / or after granulation a disintegrant is added and orodispersible tablets are prepared. The tablet has a disintegration time of less than 100 seconds, less than 90 seconds, preferably less than 80 seconds, more preferably less than 60 seconds and said disintegration time was determined according to the European Pharmacopoeia VI, Test method 2.9.1 by using a pharmaceutical disintegration tester model ZT 73 whereby 6 tablets having a surface of 1 square centimeter and a weight of 350 mg, at a compression force of 20 kN, were analyzed and mean values were calculated. The process for preparing the orodispersible tablet, its use, and the intermediate granulate are described as well.
Owner:CARGILL INC
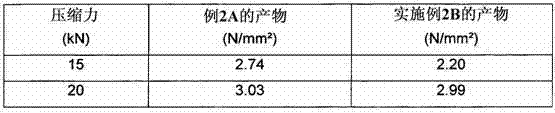
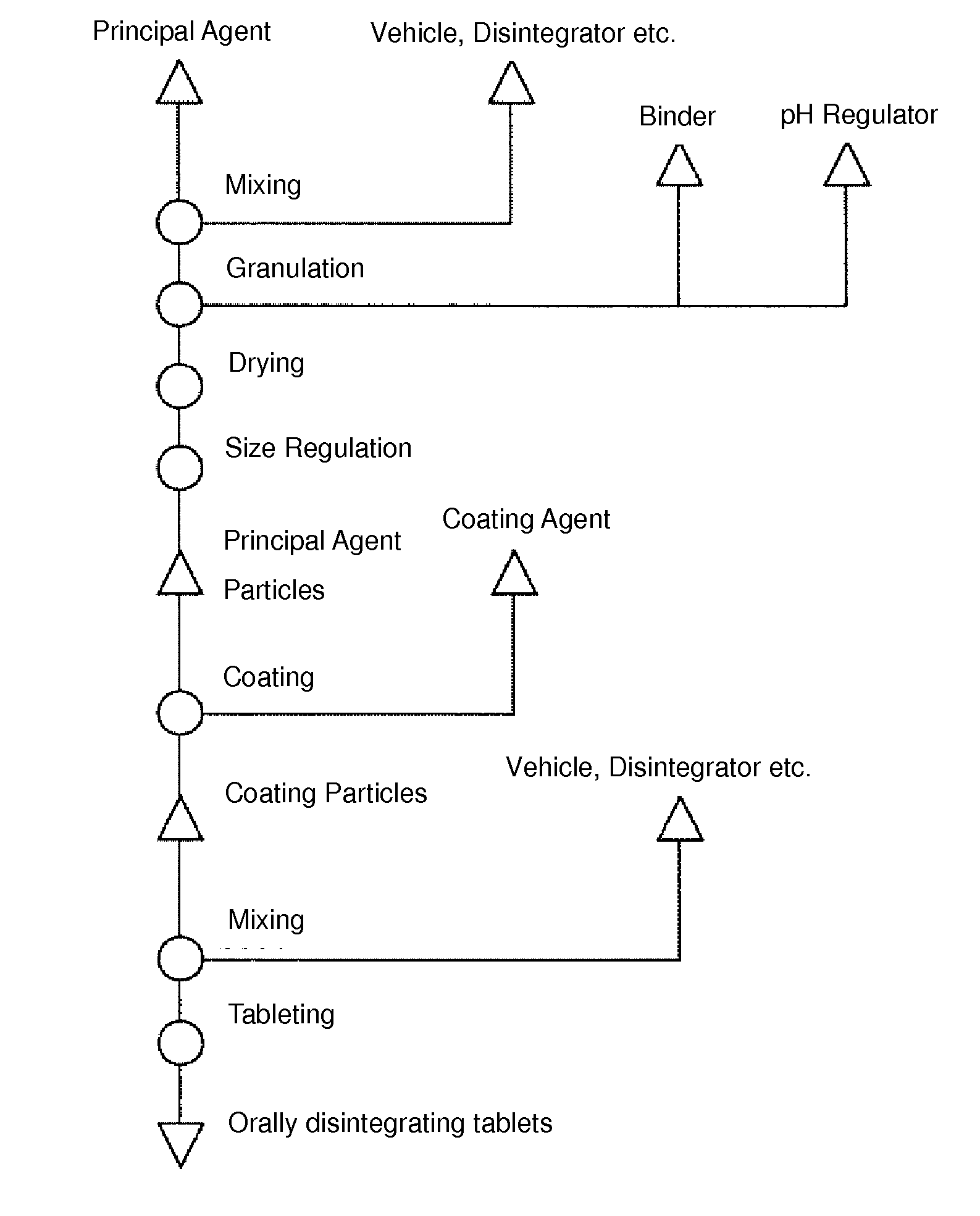
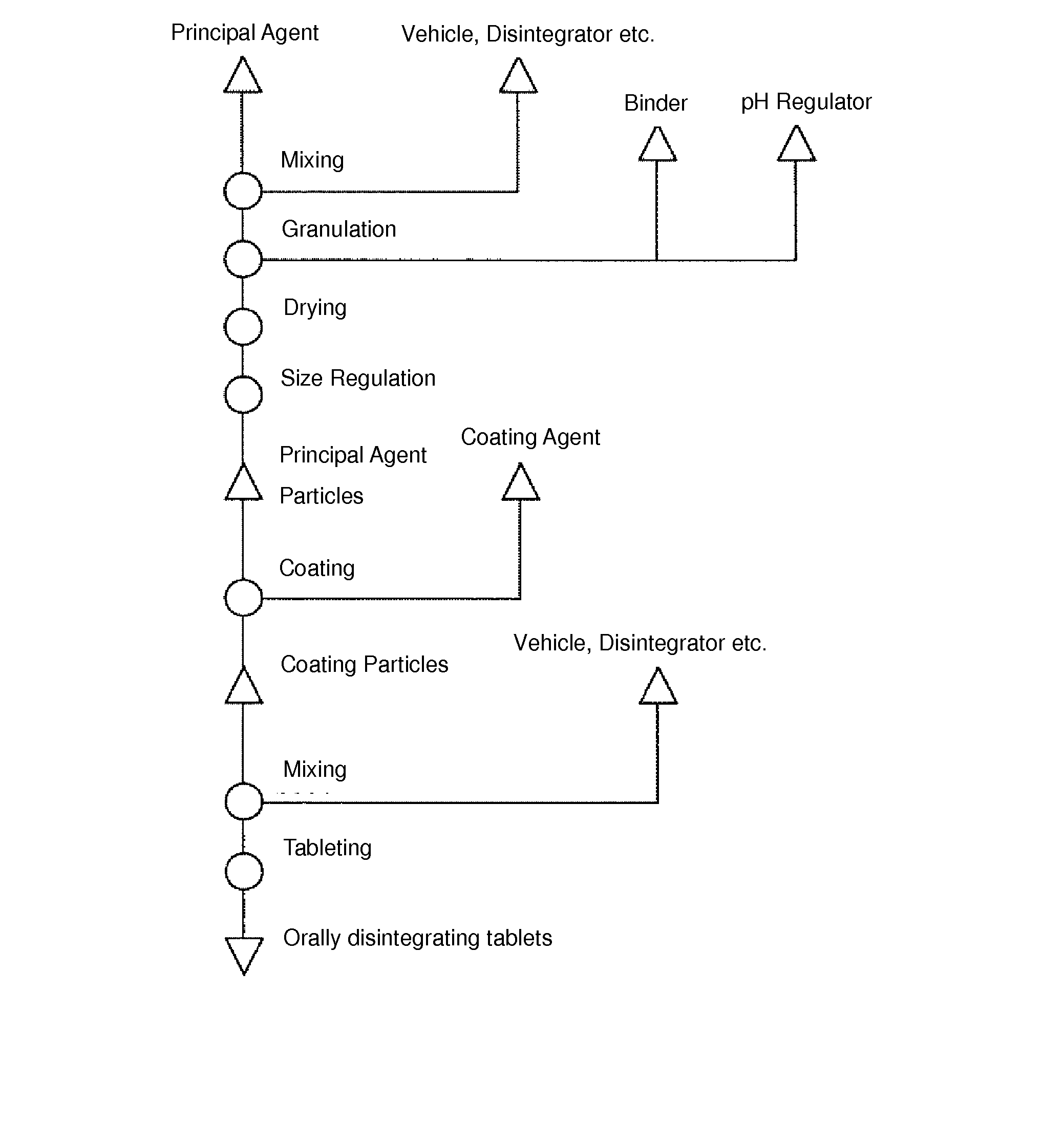
Method for Producing Orodispersible Tablets
InactiveUS20140093567A1Mask unpleasant tasteEasy to produceOrganic active ingredientsAntipyreticMedicineOrodispersible tablet
A method for easily producing orodispersible tablets that secure an eluting behavior of a principal agent in the digestive tract and mask unpleasant taste in the oral cavity is provided. Provided is a method for producing orodispersible tablets which includes mixing an additive to a principal agent to form principal agent particles as a granulation process, coating the principal agent particles with a coating agent to form coated particles as a coating process, and tableting the coated particles to form tablets as a tableting process, wherein the additive used in the granulation process contains a pH regulator, and the coating agent used in the coating process contains a pH-dependent polymer dissolving at pH 5 or more.
Owner:NIPRO CORP
Crystalline base of escitalopram and orodispersible tablets comprising escitalopram base
Owner:H LUNDBECK AS
Orodispersible tablets
InactiveUS20160310470A1Good mechanical resistanceReduce brittlenessOrganic active ingredientsNervous disorderCalcium silicateOrally disintegrating tablet
Owner:LAB LESVI SL
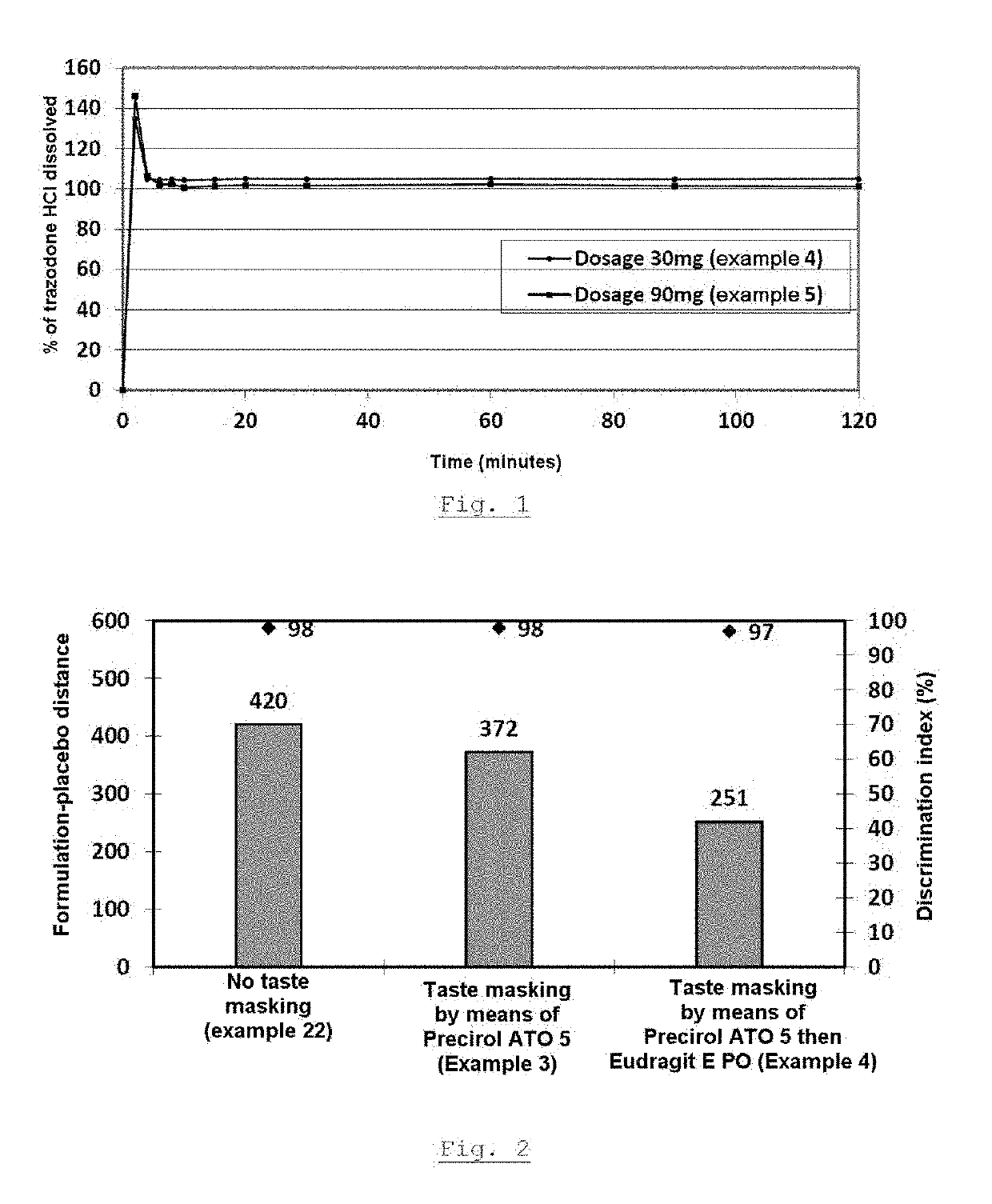
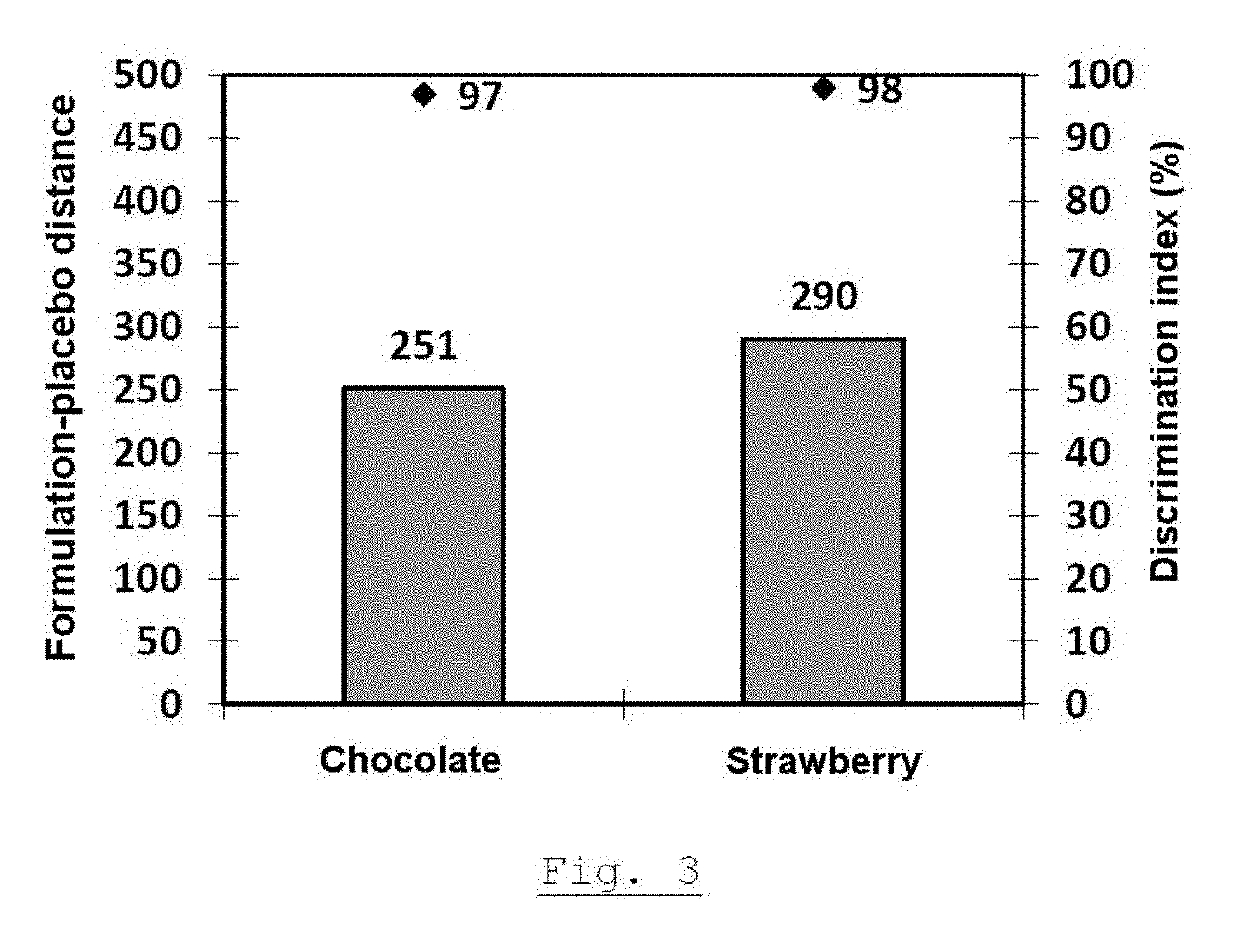
Granules of an active substance with double taste-masking technique, method for the production thereof, and orodispersible tablets containing same
The present invention relates to granules of active ingredient with double taste masking, wherein the double taste masking is achieved by a hot-melt compound selected from waxes, hydrogenated vegetable oils, fatty acids, mono-, di- and triesters of fatty acids and of glycerol, triglycerides, glycerides, polyoxylglycerides, fatty alcohols, and mixtures thereof, and a thermoplastic polymer that is soluble at a pH less than or equal to 5. The invention also relates to the method for producing these granules and to orodispersible tablets containing these coated granules.
Owner:ETHYPHARM SA
Orodispersible tablets
ActiveUS9623010B2Good mechanical resistanceReduce brittlenessBiocideNervous disorderCalcium silicateMedicine
Owner:LAB LESVI SL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com